STAINS POST-GOLDINCISION®

Content extracted from the book “Victory Against Cellulite” by Dr Roberto Chacur, Ed. AGE, 2023.
Dr. Manoela Fassina
Dr. Roberto Chacur
STAINS POST-GOLDINCISION®
Skin
The skin is a complex and dynamic organ that changes constantly. It accounts for approximately 15% of the body surface of an adult person and, in addition, regulates body temperature and protects against physical, chemical and biological agents. It consists of three main layers: the epidermis, the dermis, and the hypodermis (subcutaneous layer). Each of them is composed of several sublayers. Skin appendages, such as hair follicles and sebaceous and sweat glands, also play a global role.
Epidermis (outermost layer)
Avascular and functioning as a semipermeable barrier, the epidermis is, basically, a stratified epithelial tissue, keratinized, made up of squamous epithelial cells, which are in a constant process of renewal. Nerve endings and sensory corpuscles are located in the basal layer.
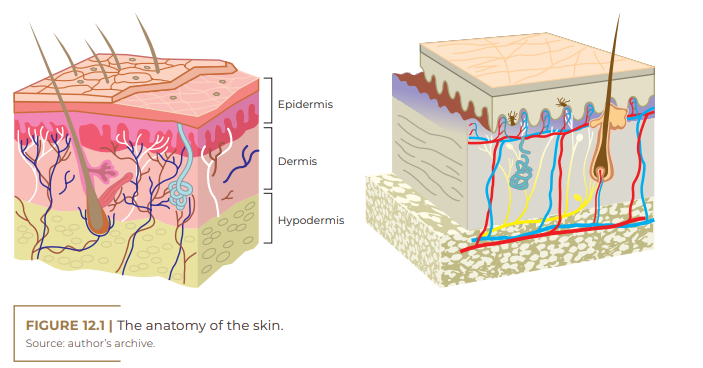
Dermis (middle layer)
The dermis is the layer of connective tissue composed of an integrated system of fibrous, filamentous and amorphous structures, in which blood vessels, nerves and epidermal appendages are located. It is in the dermis that the hair follicles, the sensitive nerves, the sebaceous glands, responsible for the production of sebum, and the sweat glands, responsible for sweat, are located (AZULAY, 2017; SBD, c2016).
Hypodermis (deepest layer)
The hypodermis is the deepest layer of the skin, presenting lipocytes, collagen with blood vessels, lymphatics and nerves. The number of cells present in this layer differs in different parts of the body. Furthermore, the distribution of fat cells also differs between men and women, as does the structure of other parts of the skin.
See Chapters
CHAPTER 1 - DEFINITION, HISTORY AND NOMENCLATURE
CHAPTER 2 - ONLINE QUESTIONNAIRE FOR CELLULITE CLASSIFICATION
CHAPTER 3 - LIPEDEMA: DESCRIPTION, DIAGNOSIS AND TREATMENT
CHAPTER 4 - ANATOMY OF THE GLUTE REGION APPLIED IN PRACTICE
CHAPTER 6 - INJECTABLE CELLULITE TREATMENTS
CHAPTER 7 - LASER-LIPO: INVASIVE TECHNOLOGY
CHAPTER 8 - OTHER CELLULITE TREATMENTS
CHAPTER 9 - BIOSTIMULATORY EFFECTS OF MICROSPHERE INJECTIONS INTO OVERLYING SKIN STRUCTURES
CHAPTER 10 - INFLUENCE OF HORMONES ON CELLULITE: WITH EMPHASIS ON ADIPONECTIN
CHAPTER 11 - GOLDINCISION®: A MULTIFACTORIAL APPROACH TO THE TREATMENT OF CELLULITE
CHAPTER 12 - STAINS POST-GOLDINCISION®
CHAPTER 13 - ADVERSE EFFECTS AND COMPLICATIONS IN GOLDINCISION®
With the collaboration of experienced medical professionals, Dr. Roberto Chacur brings together in this book an approach around the theme ranging from the genesis of cellulite, the proper method of evaluating and classifying, associated diseases and hormonal modulation to existing treatments, what really works and why the GOLDINCISION method is considered the gold standard.
SKIN PIGMENTATION
The skin is the most visible aspect of the human phenotype, and its color is one of its most variable factors. Little is known about the genetic, evolutionary and cultural aspects related to the establishment of human skin color patterns. The synthesis of vitamin D in the skin, degradation of folic acid by UVR, resistance to direct sun exposure and cultural elements are arguments on which they try to explain the phenotypic distribution of skin color in different latitudes of the planet. The color of normal human skin is mainly influenced by the production of melanin, a dense brown pigment, of high molecular weight, which becomes darker the more concentrated it is. However, exogenous yellow pigments – carotenoids – also contribute to skin color, as well as endogenous red, from oxygenated hemoglobin in the capillaries of the dermis, and endogenous blue, from reduced hemoglobin in the venules.
In humans, skin and hair pigmentation is dependent on melanogenic activity within melanocytes, the rate of melanin synthesis, as well as the size, number, composition, and distribution of particles in the cytoplasm of melanocytes, called melanosomes, in addition to nature. chemistry of the melanin they contain. Melanocytes and melanosomes have a relatively constant number in different ethnicities.
Melanocytes
Melanocytes are cells that derive from the neural crest (melanoblasts) and migrate during embryogenesis to the skin. This process begins between the tenth and twelfth week of development of the fetus for the dermis, and two weeks later for the epidermis, where they differentiate into melanocytes, which around the sixth month of fetal life are disposed at the dermal-epidermal junction (HEARING; TSUKAMOTO, 1991). Several cytokines and growth factors are accepted to support the differentiation of melanoblasts to melanocytes and their migration.
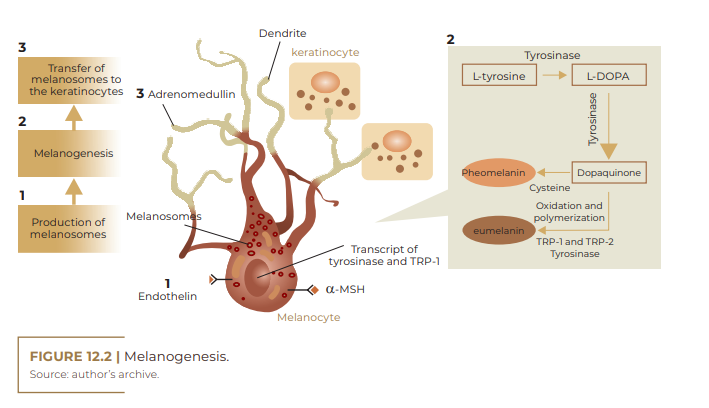
The association between a melanocyte with 36 keratinocytes constitutes the melano-epidermal unit (DUVAL et al., 2002). Melanocytes reach specific sites, among them: dermis, epidermis, hair follicles, uveal tract of the eye, vestibule and endolymphatic sac of the ear, leptomeninges of the brain. According to HEARING; TSUKAMOTO (1991), melanocytes synthesize melanin within organelles, called melanosomes, which can vary in size, number and density, and are subsequently transferred to keratinocytes and hair bulbs. Melanocytes are influenced by a variety of extracellular factors that determine the initiation of synthesis and the type of melanin to be produced. The synthesis and distribution of melanin in the epidermis involves several steps: transcription of proteins necessary for melanogenesis, melanosome biogenesis, sorting of melanogenic proteins within melanosomes, transport of melanosomes to the edges of melanocyte dendrites, and transfer of melanosomes to keratinocytes (PARK et al., 2008). Inside the melanosomes, there are three enzymes that are absolutely necessary to synthesize the various types of melanin. While tyrosinase is responsible for the critical step of melanogenesis (biosynthesislimiting step, tyrosine hydroxylation), tyrosinase-related protein-1 (TRP-1), and dopachrome tautomerase (DCT) are more involved with the modification of melanogenesis. melanin in different types. In addition to these, melanosomes contain other melanocyte specific proteins, which have structural functions or are involved in pH regulation within melanosomes, such as P protein or membrane associated transporter protein (MATP) (COSTIN; HEARING, 2007).
Melanin
The pigmentation of skin, eyes and hair depends on a wide variety of factors that influence melanocyte function at various levels. There are a large amount of genes that affect all levels of melanogenesis, directly or indirectly. Many of these genes encode proteins that are located in melanin granules, which play an important role in the structure and functioning of these organelles, both catalytic function in melanin synthesis and structural function in the integrity of melanosomes. Many of these genes have been implicated in several genetically inherited pigmentation disorders (HEARING, 2006). The pigment melanin has a wide range of important physiological functions, such as protection of underlying tissues exposed to ultraviolet (UV) radiation, temperature control and production of adaptive coloration in the skin (PROTA, 1980), its main role in human skin is to attenuate the penetration of UV rays in deeper proportions, such as in blood vessels of the dermis (SLOMINSKI et al., 2004). Furthermore, it is the major determinant of human skin color, synthesized from L-tyrosine. It is composed of a pigment that varies from brown to black, eumelanin, and another that contains sulfur that varies from red to yellow, pheomelanin (DUVAL et al., 2002), while Slominski et al. (2004) propose that there are several types of melanin, such as eumelanin, pheomelanin, neuromelanin and a mixture of melanic pigments, characterizing them as polymorphs and multifunctional biopolymers.
Eumelanin and pheomelanin are tightly associated with proteins, but exhibit differences in chemical and physical properties in protecting against UV radiation. Eumelanin is considered a photostable, photoprotective polymer, insoluble in most solvents; on the other hand, pheomelanin is soluble in alkalis, photolabile, photosensitizing, for producing superoxide and hydroxyl radicals and hydrogen peroxide after solar radiation (DUVAL et al., 2002; SLOMINSKI et al., 2004). The sex hormones estrogen and progesterone have been reported to interact with melanocytes, although the mechanism and differentiation have not been clarified. It is believed that the increase in pigmentation often seen in pregnancy is a phenotypic effect of estrogen production (HEARING, 2006).
Environmental temperature variations can influence human skin biology, consequently epidermal keratinocytes exposed to heat, cold or oxidative stress, result in the induction of skin inflammation; for example, heating leads to the formation of interleukins 1-alpha, prostaglandins E2 (ALLAPPATT et al., 2000). Many chemical compounds have shown inhibitory effects on melanogenesis by inhibiting the enzymatic activity of tyrosinase, but effects related to gene expression, protein degradation, glycosylation, melanosome transfer and regulation of cellular signals have also been reported in the control of melanogenesis (SOLANO et al. , 2006).
PIGMENTATION DISORDERS (DYSCHROMIAS)
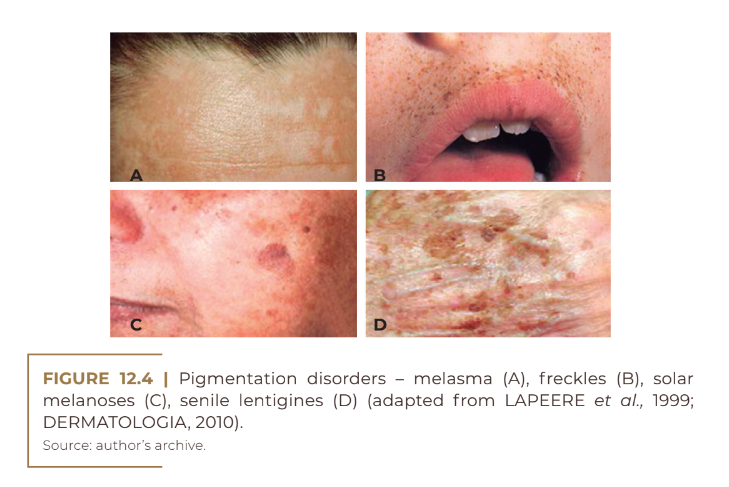
Within this order of skin colorations, there are disorders of the pigmentary system, which result in hyperpigmentation and hypopigmentation problems. Skin hyperpigmentation is the most common complaint among patients who consult a dermatologist in search of restoring a homogeneous skin tone. They can be divided into diffuse and circumscribed, linear and reticulated hyperpigmentation.Some types of hyperchromic spots are: melasma (Figure 12.4A), freckles (Figure 12.4B), chloasma, solar melanoses (Figure 12.4C), senile lentigens (Figure 12.4D), Riehl’s melanosis, Civatte’s polychyloderma, photosensitivity melanodermatitis , residual melanoderma, periorbital hyperpigmentation, post-inflammatory hyperpigmentation.
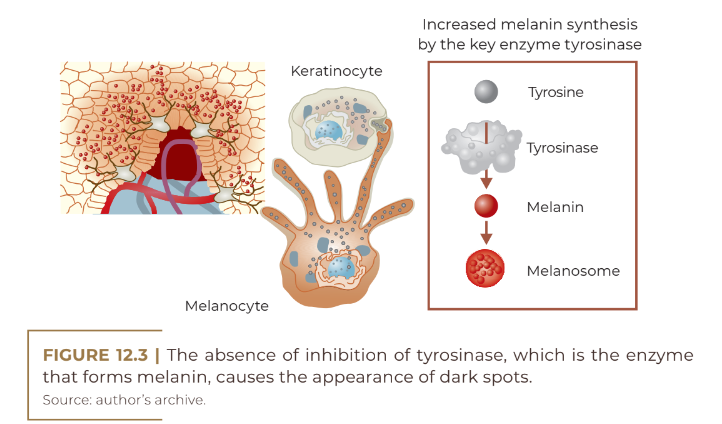
Hyperpigmentation of the skin can result from an increase in the amount of melanin, an increase in the size of melanosomes and an increase in the number of melanocytes, which can occur due to many factors such as aging, pregnancy, endocrine disorders, treatment with sex hormones and exposure in the sun to varying degrees. Epidermal lesions occur due to an increase in the number of melanosomes or the rate of melanogenesis and are more brownish in color. On the other hand, dermal hyperpigmentation occurs due to increased deposition of melanin in the dermis, retained by macrophages, or due to increased melanin production in dermal melanocytes and presents with a gray to blue-gray color. And, finally, mixed hyperpigmentation, which affects the epidermis and dermis.
Post-inflammatory hyperpigmentation is the most common type of acquired hyperpigmentation. It is more recurrent in people with higher phototypes and is the result of inflammation that may have been caused by any acute inflammatory event that, through various mechanisms, including direct stimulation of melanocytes by inflammatory mediators such as interleukin-1-α , endothelin-1 and reactive oxygen species, generated by skin damage. Furthermore, damage to epidermal cells can release endocrine pigmentation inducers such as the hormone α MSH. All these factors generate hyperpigmentation. The melanin produced during the inflammatory event can enter the dermis, generating internalization by macrophages of this excess pigmentation; thus, the macrophages are dammed in the dermis for long periods (ORTONNE, BISSETT, 2008). The colors of the lesions vary from brown to black to gray and usually follow the distribution of the primary dermatosis.
Hyperchromia due to hemosiderin deposition results from the rupture of blood vessels, which causes the development of edema and the accumulation of hemoglobin in the treated region. The ferric portion of hemoglobin binds to a protein part, forming ferritin (portion of iron available in the body). The degradation of ferritin releases ferric ions, which, disconnected from the protein part, become toxic, and react with oxygen, producing free radicals; these, after a complex process, are destabilized, generating an oxidative chain process and becoming reactive. In order to avoid this process, the body protects itself by forming hemosiderin, which is a kind of storage for the crystallized iron ion accumulated in cells, mainly in the endothelial reticulum. The result is the formation of an insoluble, non-toxic compound that is deposited in the dermis. It is histologically verifiable 24 to 48 hours after the extravasation of blood, with a peak between the 4th and 5th day.
Histological studies demonstrate that brownish-brown pigmentation is caused by alteration of the dermal color by hemosiderin. The pigment is predominantly in the superficial dermis, and may sometimes be present in the periaxial regions or in the middle dermis. Some authors believe that the oxidation of the iron present in the lesions would lead to the formation of radicals, which, due to their toxicity, would stimulate the melanocytes, worsening the lesions. It was found that iron was deposited in the extracellular matrix between collagen fibers and within granules of Langerhans cells, giving the skin a brown appearance, characteristic of hemosiderin (Quezada Gaón et al.).
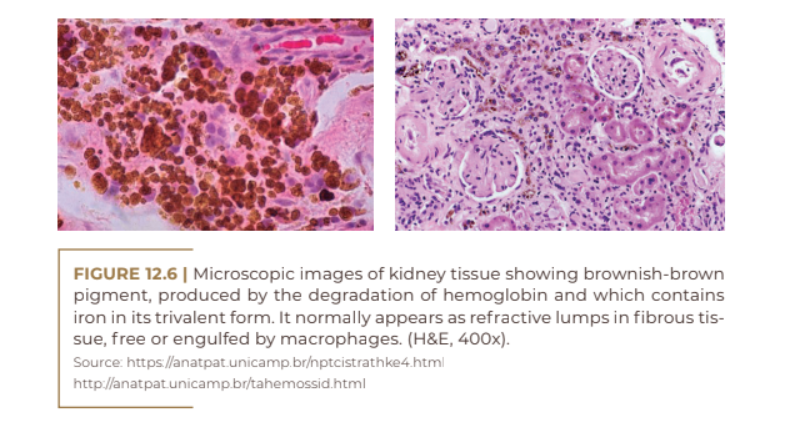
The Goldincision® procedure, based on collagen biostimulation and disruption of the fibrosis septum; in addition to releasing the skin, the procedure promotes the formation of a collection of blood (hematoma) by breaking small blood vessels below the treated lesion. This hematoma, in addition to the trauma and the inflammatory process, will help in the production of new and better-organized collagen, collagen that fills the treated area, giving the skin a more uniform relief appearance, in addition to making it firmer. Residual macules are a cause of distress for many patients and seem to be the result of hemosiderin deposits associated with melanic hyperpigmentation, as it is believed that there is melanocytic activation secondary to the deposition of ferric pigment in the dermis.
ALERT FACTORS
Some factors serve as a warning during the anamnesis for those patients who will have a greater chance of developing the spots. Among them we can mention genetics – higher phototypes tend to stain more than lower phototypes. Smoking, alcohol, use of vasodilators, chemotherapy and antipsychotics are factors that can contribute to this process by stasis of blood vessels, favoring the color change in the region.
Hormone replacement therapy and the use of contraceptives, as well as antibiotics from the tetracycline class, can lead to increased melanin production. On the other hand, diseases that cause fluid retention, such as thyroid disease, kidney disease, heart disease and lung disease, tend to keep the hematoma in place for a longer time, favoring the formation of hemosiderin pigment. And vitamin K deficiency is also linked to increased bleeding time.
Patients using medications containing iron, it is recommended that they avoid ingesting these medications, as well as reducing the intake of this mineral 30 days before the procedure. Ideal treatment should include discontinuing triggering factors when identified.
TREATMENTS
The good news is that studies have shown that these spots resolve spontaneously, without the help of creams, lasers or any treatment hatsoever. It is estimated that 80% of spots disappear completely after period of 6 to 24 months. However, the sooner therapy is instituted to resolve the inflammatory process, the better and earlier the result of treating the spots will be. First-line therapy consists of the use of topical whitening agents, and technologies may be associated, including photoprotection, before, during and after the therapeutic process. Treatment for stains is often time consuming, and results may vary according to the intensity of the injury. Several treatments are used to treat this type of hyperchromia, such as, for example, the use of depigmenting topics, percutaneous collagen induction, the use of chemical peelings, microneedling, laser treatment and intense pulsed light (Bomfim et. al, 2022). However, it is important to always be aware of the potential that the treatment itself has to cause or aggravate PIH, causing irritation.
Whitening agents
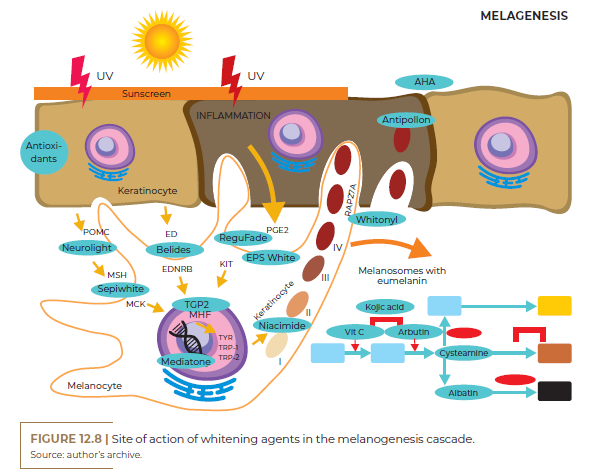
Whitening agents have active principles that act through different mechanisms of action. An important point is that they are all linked to interference in the production or transfer of melanin, either by inhibiting tyrosine biosynthesis, inhibiting melanin formation, interfering with the transport of melanin granules, chemically altering melanin, selectively destroying melanocytes and inhibiting the formation of melanosomes and alteration of their structure. Therefore, treatments for lightening hyperpigmentation must contain associations of two or more agents with different mechanisms to produce a synergistic effect. In addition to its mechanism of action, other parameters related to cytotoxicity, solubility, cutaneous absorption, penetration and stability of treatment agents must be considered.
It is known that the treatment of dyschromic skin is somewhat difficult, as many effective compounds in the treatment have irritating properties and can, in certain cases, promote desquamation. Satisfactory results are not achieved immediately, as depigmentation is gradual.
• UVA-URSI EXTRACT: rich in natural Arbutin (Beta-Arbutin). Capable of causing discoloration of already formed melanin and inhibiting the formation of new melanin. It inhibits the darkening process, effectively reduces existing pigmentation, in addition to having the ability to inhibit tyrosinase by 100% and naturally degrade the melanin present in the skin. The action of uva-ursi is a little slower, but cumulative (not reversible) when compared to hydroquinone, that is, when achieving the desired lightening, the stains will not reappear afterwards (FÁBIO BORGES).
• HALOXYL: is an active composed of matrikines, which stimulate the synthesis of extracellular matrix (ECM) components. Chrysin and nhydroxysuccinimide act as bilirubin and iron chelators, respectively, decreasing local pigmentation.
• THIOGLYCOLIC ACID: or mercaptoacetic acid, widely used for the treatment of persistent hematomas. Its affinity for iron and for the treatment of persistent hematomas is similar to that of apoferritin, having the ability to chelate iron from hemosiderin, as it has a thiol group (SH). It is used topically at home in a concentration of 5 to 10%. Guide a thin layer for 20 to 30 minutes and then remove.
• ARBUTIN: natural and stable derivative of hydroquinone, linked to a D-glucose (sugar). Widely used for the depigmenting treatment of sensitive skin, high phototypes and sensitive areas (VANZIN et al., 2011). It works by blocking the action of the tyrosinase enzyme, preventing the production of melanin at the point of application. It is less likely to cause irreversible hyperpigmentation. The potentiated form α-Arbutin causes a faster, more efficient and safer depigmenting effect. The usual concentration of α-Arbutin ranges from 0.2 to 2%.
• KOJIC ACID: obtained from the fermentation of rice. It has an inhibitory effect on tyrosinase, by chelating copper ions and, consequently, decreasing melanin synthesis. It also induces a reduction in the synthesis of eumelanin in hyperpigmented cells. It is an excellent depigmenting alternative for the treatment of higher phototypes, especially when associated with phytic acid. The usual concentration of kojic acid can vary from 1 to 5%.
• SEPIWHITE MSH: decreases the activity of the enzyme tyrosinase, reduces the production and fixation of eumelanin in keratinocytes. Blocks receptors on melatropin hormone binding. This hormone is directly involved in stimulating the tyrosinase enzyme in the synthesis of melanin (especially eumelanin), and is also responsible for favoring the fixation of the pigment formed in keratinocytes, increasing skin luminosity (Vanzin et al.).
• ANTIPOLLON: an interesting depigmenting agent, as it acts on the adsorption and elimination of already formed melanin, and can be associated with other substances, except fatty acids. It may be indicated in the depigmenting treatment of pregnant women or patients intolerant to the use of conventional depigmenting agents.
• PHYTIC ACID: inositol hexaphosphate, a substance present in cereals that exert an inhibitory action on tyrosinase. It also has anti-inflammatory, antioxidant, moisturizing and chelating actions. In addition to being indicated for the treatment of hyperchromia resulting from melanin deposits, due to the chelating action that the acid has, it is especially indicated for the treatment of hyperchromia caused by hemosiderin deposits, due to its potent chelating action for the iron ion.
• VITAMIN C: ascorbic acid, which lowers chemical stability in aqueous solutions, easily oxidizing in gels, cream gels or oilin-water emulsions (PEYREFITTE, 1998). Investments were made in obtaining vitamin C derivatives that performed the same functions and, on the other hand, had greater chemical stability and cutaneous penetration at more effective levels, so that pharmacodynamic functions would not be compromised (GONÇALVES, 2002). Thus, vitamin C can be presented in different ways in products with the purpose of whitening, acting on the synthesis of melanin (tyrosinase inhibition), an antioxidant, and on the synthesis of collagen, improving the appearance of the epidermis.
• BELIDES: Belis perennis is a depigmenting active whose effect occurs even before melanin is formed, as it decreases the action of endothelin 1, an inflammatory mediator produced in keratinocytes and which activates melanocytes. Another mechanism is the decrease in the binding of α-MSH (melanocyte stimulating hormone) in melanocytes and consequent decrease in the production of eumelanin. It also reduces the transfer of melanosomes formed in the melanocyte to the surrounding epidermal cells, reducing their pigmentation. It is indicated in the treatment of hyperchromia resulting from melanin deposits, as the only depigmenting active or associated with other products, such as hydroquinone. Its usage concentration ranges from 2.0 to 5.0%.
• VITAMIN K: Vitamin K is essential for the synthesis of blood clotting factors. It is on the list of substances that should not be part of the composition of cosmetic products since 2010.
• ALGOWHITE: a depigmenting agent that acts by reducing the activity of endothelin 1 in melanocytes and by inhibiting the tyrosinase enzyme; it accelerates cell differentiation and has an antioxidant effect.
• WHITESSENCI: this extract allows the lightening of melanin derived hyperchromias by reducing melanosome firecytosis, thatis, it decreases the fixation of melanin formed in keratinocytes. Contains whitening action proteins such as jacalin and artocapin.
• HYDROQUINONE: phenolic compound that inhibits the enzymatic oxidation of tyrosine and other metabolic processes in melanocytes. It is a low-cost depigmenting agent with a rapid whitening response. Due to side effects, hydroquinone is banned in several countries.
• AZELAIC ACID: Has several mechanisms by which it depigments the skin, including tyrosinase inhibition, as well as selective cytotoxic and antiproliferative effects on abnormal melanocytes through inhibition of DNA synthesis and mitochondrial enzymes. Available formulations include a 15% gel or 20% cream.
• CISTEAMINE 5%: is one of the latest skin lightening products. The skin lightening effect is believed to be due to its inherent antioxidant properties causing a lightening effect on the stratum corneum. It is hypothesized that cysteamine reduces melanin production by inhibiting the main melanogenic enzymes, tyrosinase and peroxidase, as well as the chelating copper ions required in melanogenesis.
• NIACINAMIDE: physiologically active derivative of vitamin B3 (niacin), significantly decreases melanosome transfer to keratinocytes without inhibiting tyrosinase activity or cell proliferation. It may also interfere with the cell signaling pathway between keratinocytes and melanocytes to decrease melanogenesis. One of the advantages of niacinamide is that its stability is not affected by light, humidity, acids, alkalis or oxidants. Topical 2 to 5% niacinamide has shown some effectiveness when used alone or in combination.
• TYROSINASE ANTAGONIST: assets that structurally mimic tyrosine, occupying space in the tyrosinase enzyme, preventing its action. Eg: arbutin, hydroquinone, ursi grape.
• ANTIOXIDANTS: they hinder the process of tyrosine oxidation, reduce the inflammatory process, fight free radicals, reduce aggressions to cell membranes. Examples: Vitamin C, Ferulic Acid, Vitamin E.
• COPPER ION CHELATORS: tyrosinase is a metalloprotein, therefore it needs metals as a cofactor. Copper is a fundamental metal for the functioning of this enzyme. By chelating this metal, tyrosinase has difficulties in acting, reducing this step of melanogenesis. Examples: kojic acid, phytic acid.
• GLUTATHIONE BOOSTER: Glutathione is an important endogenous antioxidant enzyme that diverts melanin synthesis towards the formation of pheomelanin (yellow), decreasing the production of eumelanin (brown and black). Ex.: NAC, cysteamine.
• TRP-2 INHIBITOR: enzyme responsible for accelerating the formation of black eumelanin. Eg: albatin.
• MELANOSOME TRANSFER INHIBITOR TO KERATINOCYTES: Prevents mature melanin-filled melanosome from being delivered to keratinocytes to pigment their cytoplasm. Eg: niacinamide, Whitessence.
• RAB27A INHIBITOR: this glycoprotein is essential for transporting the melanosome through the actin filaments to be transferred to the keratinocytes. Ex.: Whitonyl.
• FORMED MELANIN ADSORBENT: disperses and adsorbs the formed melanin pigment in keratinocytes. Ex.: Antipollon HT.
• CHEMICAL EXFOLIANTS: remove the melanin-impregnated stratum corneum in the outermost layer of the skin. Example: alphahydroxy acids.
• TYROSINASE SYNTHESIS INHIBITOR: inhibition of genes that activate melanogenesis via the melanocyte nucleus. Ex.: Mediatone or O.D.A. White.
• MITF-DERIVED TRANSCRIPTION FACTOR INHIBITOR MITF: transcription factor important for initiating melanogenesis. Ex.: TGP2 peptide, Whiteris G.
• ALPHA MSH ANTAGONIST: main activator hormone of melanogenesis. Ex.: Sepiwhite, IluminScan, Delentigo.
• ENDOTHELIN ANTAGONIST: Endothelin-1 is the main mediator of melanogenesis sensitive to activation by UV radiation. Ex.: Belides, AlgoWhite.
• SCF AND GM-SCF ANTAGONIST: Keratinocytes are able to stimulate melanogenesis through mediators and colony stimulating factors. Ex.: Regu-Fade.
• PGE-2 INHIBITOR: Inflammatory prostaglandins are effective stimulators of melanogenesis, particularly in post-inflammatory hyperpigmentation. Ex.: EPS White, Physasun.
• POMC BLOCKER: Pro-opiomelanocortin is a precursor to the hormone alpha-MSH, which activates melanogenesis. Ex.: Neurolight.
• SUNSCREEN: the most efficient blocker of UV radiation on the skin.
Peeling
Chemical peeling operations are among the most common cosmetic procedures in medical practice and have been used for decades because they are simple and inexpensive. It is defined as the application of chemical agents of varying strength to the skin, which results in the controlled destruction of the epidermis and dermis (Carrer et al., 2008). The induced exfoliation is followed by dermal and epidermal regeneration of the adjacent epithelium and skin appendages, which results in improved surface texture and skin appearance (Bonfim, 2022).
Chemical peeling operations are classified, based on the depth of penetration, into superficial (epidermis – papillary dermis), medium (papillary to upper reticular dermis) and deep (middle reticular dermis) peels. In pigmentation disorders, we normally use superficial and medium peeling operations. Glycolic acid (GA) is used as a superficial or medium-depth peeling because it is an exfoliative agent that causes epidermolysis with skin desquamation due to reduced adhesion of corneocytes and obstruction of keratinocytes in the stratum granulosum. Similar to other α-hydroxy acids, it leads to thickening of the epidermis and dermis, with increased synthesis of collagen and mucopolysaccharide, and dispersion of melanin. GA peeling agents are available in concentrations from 20 to 70%.
Penetration depth and intensity of GA peeling agents increase with concentrations and exposure time. This agent must be neutralized with an alkaline solution such as baking soda or normal saline to stop its exfoliative effects. Lactic acid (LA) is used alone or in combination with other peeling agents. Its whitening effects on the skin are due to decreasing melanin synthesis by directly inhibiting the tyrosinase enzyme. Jessner’s solution (JS) is a superficial peeling agent commonly used with other peels to increase penetration. Its mechanisms of action are specific to each ingredient, but it is generally proposed to break bridges between keratinocytes. Kojic acid (KA) is a copper chelating agent, and its whitening properties lie in its ability to inhibit the enzyme tyrosinase. Available in concentrations from 1 to 4% and often used in combination with GA or other bleaching agents (arbutin, aloesin, soy extract, etc.) to increase penetration and effectiveness. It can be used before and after peeling to prevent and treat post-inflammatory hyperpigmentation. Thioglycolic acid (TA) in stronger concentrations, such as 20%, is used in the form of peelings, which must be repeated fortnightly. The number of sessions required generally varies between 3 and 6 applications. The skin almost does not flake off, and may remain reddish in the first 12 hours. The use of sunscreen is essential after the sessions.
Technologies
Lasers and light sources can be an effective complement to the therapy of choice or an alternative to treatment failures with bleaching agents. Several lasers have been used to treat pigmentary lesions, the main ones being ruby (694 nm) and alexandrite (755 nm). Higher phototypes are more likely to develop adverse reactions, especially additional PIH after laser treatment.
The Nd LASER: YAG with a wavelength of 1064 nm is most effective for removing pigment tattoos or black and blue inks. It can also have its frequency doubled, emitting a wavelength of 532 nm, which is more absorbed by melanin, being indicated for the treatment of superficial vascular lesions (Roh; Chung, 2009; Agne, 2009; Horibe, 2000; Alam; Gladstone; Tung, 2010; Chavantes, 2009; Goldberg, 2007). According to Agne (2009) and Cameron (2009), in low power laser therapy, important therapeutic effects predominate, which can be observed clinically, in particular local analgesia, reduction of edema, anti-inflammatory action and stimulation of healing of difficult-to-heal wounds. The direct effects, triggered by the absorption of energy, are limited to the point of application, the depth of penetration and the time the application lasts (Nunes et al., 2013).
Indicated for the treatment of superficial vascular and pigmentary alterations, the Q-switch laser has a wavelength transmitted by water, although it penetrates superficially into the skin, being absorbed at a distance smaller than 0.1 mm in melanin and 0.5 mm in blood. Intense pulsed light (IPL) differs from lasers in general because it has a polychromatic light that emits a broad spectrum of wavelengths, generally in the range of 400 to 1,200 nm and the incoherent light factor. Energy, which is emitted in all directions, spreads out. Focusing and directing light is done by means of mirrored surfaces placed behind the lamps. In this way, the application is smoother and of lower intensity than the laser (Nuno, 2009). IPL systems emit single, double or triple pulses, with variable intervals, allowing tissue cooling from 2 to 25 milliseconds in duration. Longer wavelengths penetrate deeper into the skin, thus increasing the destruction of deep vessels, while longer pulse durations heat larger vessels more slowly, preventing vascular disruption. (Kede, Sabatovich, 2009; Sampaio; Rivitti, 2000; Alam; Gladstone; Tung, 2010).
IPL technology is used effectively to treat a variety of vascular and pigmented disorders, as well as other indications. However, one of its main limitations lies in the treatment of patients with higher phototypes, requiring the professional to determine the energy fluency according to the Fitzpatrick table.
The expression light emitting diodes (LED) refers to semiconductor diodes subjected to an electric current, which emit the light used for phototherapy, with a wavelength ranging from 405 nm (blue) to 940 nm (infrared). Red light is most suitable for treating superficial tissues, at a depth of 5 to 10 mm, such as the skin and subcutaneous tissue. Applicators that release blue light are best suited for treating even more superficial tissue, such as exposed skin or soft tissue (Jedwab, 2010; Cameron, 2009).
LED emitters produce low-intensity light, which may appear to be one color, but is neither coherent nor monochromatic. The emitted light is non-directional and spreads widely. LEDs provide a more diffused light, which is better suited for treating larger, more superficial areas with a higher frequency range. They are of low power individually (Cameron, 2009; Nuno, 2009; Kalil, 2011). Its action takes place by direct and intracellular stimulation, specifically in the mitochondria, reorganizing the cells, inhibiting actions and stimulating other results in the so-called photobiostimulation or photomodulation effect (Jedwab, 2010). The blue LED has a moisturizing effect and can be used for treatment involving hyperpigmentation due to vascular alteration.
Oral treatment
The treatment of hyperchromia still has limitations and there is some evidence supporting the effectiveness of oral therapy.
• Polypodium leucotomos: beneficial properties attributed to the presence of several compounds with antioxidant and photoprotective properties. Orally, P. leucotomos provides some degree of protection against the harmful effects of ultraviolet radiation, thus helping to minimize the photoaging effects of sunlight, including hyperpigmentation and textural changes.
• Tranexamic Acid: This is an antifibrinolytic drug prescribed to treat bleeding and is also used off-label to treat and/or prevent PIH. It is used orally 500 mg per day. It is contraindicated in patients with hypercoagulable conditions, renal failure, vision disorders, pregnancy, breastfeeding, or on hormonal therapies. The exact mechanism of TXA in reducing melanogenesis is unknown.
• Hydroxytyrosol: hydroxytyrosol (Oli-Ola®) has antioxidant properties superior to those of vitamins C and E. It has chemopreventive effects against damage caused by UV radiation. It is marketed as an oral peeling agent at concentrations of 300 mg daily in single treatments or together with other whitening agents, with the aim of reducing skin hyperpigmentation.
• Pinus pinaster (pycnogenol): has antioxidant and anti-inflammatory properties and therefore eliminates free radicals. Its activity consists of inhibiting the biosynthesis of tyrosinase, causing interference in the formation of melanin. It has been used due to its inhibitory effect on pigmentation, with the aim of improving the appearance of the skin and progressively reducing the area and intensity of hyperchromias. Studies prove it to be more potent than vitamins E and C, with the ability to increase the endogenous antioxidant system, being marketed in concentrations of 75 to 100 mg.
• Grape seed extract (proanthocyanidin): contains a powerful antioxidant, and its oral intake, 67mg 3x a day, for 6 months, was considered beneficial in patients with melasma, requiring further studies on the benefit in IPH.
• Glutathione: is a compound produced in the body and acts as a strong antioxidant. It has whitening activity through several mechanisms: change in the production of pheomelanin over eumelanin; tyrosinase inhibition; and quenching ROS and free radicals, which influence tyrosinase activation. In a clinical study, 50 mg of glutathione lozenge was shown to alleviate or moderately reduce hyperpigmentation in 90% of subjects (Handog et al., 2016). Although glutathione is often marketed as a safe treatment, studies on its use for skin lightening are lacking, with most studies having limitations including small sample sizes, short study durations, short follow-up periods, and the lack of bioavailability. Additionally, IV use has been associated with several complications, including Stevens-Johnson syndrome, abdominal pain, kidney dysfunction, brain toxicity, and liver dysfunction.
• Arnica: among herbal and homeopathic medicines, arnica montana is one of the most used, given its potent anti-inflammatory activity, being indicated, above all, for reducing edema and relieving pain resulting from tissue trauma. They are also attributed healing, antiseptic, antimicrobial, fungicidal, antihistamine, cardiotonic and anticoagulant properties.
Alternative treatments
Some other treatments can be associated to optimize the response to conventional treatments. They include:
• Carboxytherapy: the mechanism of action of carboxytherapy is mechanical and pharmacological. The mechanical effect occurs due to the needle and gas entry trauma, which generates an inflammatory process, with the consequent migration of fibroblasts to the site, initiating collagen synthesis, tissue repair, increased oxygen exchange at the site, improving the irrigation and cellular nutrition (Borges, 2010; Roh; Chung, 2009). Indications of the carboxytherapy technique are pathologies that benefit from increased circulation and oxygenation. Carboxytherapy has brought good results in most patients with complaints of hyperchromia. In patients who do not obtain a minimum of improvement until the third session, the treatment is suspended. The application is carried out in such a way as to promote tissue displacement, with a flow of 80 ml/min to 150 ml/min and monthly frequency of sessions.
• Phototherapy: modifies cellular activity, using a light source without thermal effect, laser and/or LED, with the aim of improving pigmentary changes, removing pigments, increasing microcirculation and improving the elimination of toxins.
• Microcurrent: viable alternative in the treatment of PH. It is a type of electrostimulation. The effects: skin revitalization, in- crease in ATP synthesis and collagen production, skin whitening, stimulation of skin microcirculation, resulting in better nutrition and tissue oxygenation, lymph mobilization, improving local cir- culation. Microcurrent results are cumulative.
• Platelet-rich plasma (PRP): PRP is an autologous compound, whose platelet concentration is 3 to 5 times greater than the normal plasma concentration. In addition to the high concentration of platelets, it is rich in growth factors, which are secreted by platelets and act in the healing process. According to Mehryan et al., in their studies, when it comes to the effectiveness of using PRP to improve HPO, there is a significant result in color homogeneity.
• Use of hyaluronidase: can help treat hematomas in the gelatinous (clotting) and consolidation phases, thus reducing the risks of complications, patient downtime and the appearance of post- hematoma spots.
• Percutaneous collagen induction technique: also known as microneedling, it can be included in our therapeutic arsenal in the treatment of hyperchromia, especially when it proves to be resistant to conventional treatments. The number of sessions with an interval of 30 days depends on the intensity of the HPI, with no limit to the number of interventions. In addition, each intervention offers a gain in pigment reduction and improvement in skin quality, even more so when associated with drug delivery.
• Sclerotherapy: PMMA induces neocollagenesis and neovascularization due to the inflammatory pattern of the foreign body-like reaction. In some patients, the formation of small vessels, the telangiectasias, can be observed, which represent veins smaller than 1 millimeter in diameter that develop just below the skin and even the stain, usually reddish or purplish in color. Although they pose no threat to the individual’s health, they are undesirable both because they are unsightly and because they are also possibly associated with the stain itself. The objective is to cause destruction of the endothelium, so that it obliterates the light of the vessels themselves and, in this way,
no more blood passes through there. Over time, the organism recognizes this vessel as non-functioning and absorbs it, making it no longer visible.
Promising treatments
The need and demand for newer, safer, and more effective treatments for the most diverse hyperpigmentation disorders is always paving the way for continual exploration of treatment options. Several compounds and combinations are currently being tested and have shown promising results in early stages of clinical trials and are being considered for fur- ther evaluation.
Solid lipid nanoparticles
One area that has shown promising results is the use of alternative vehicles for drug delivery. The use of solid lipid nanoparticles can increase the bioavailability and topical stability of drugs. For example, hydroquinone prepared for solid lipid nanoparticles showed improved efficacy when compared to standard topical hydroquinone. Furthermore, other drug vehicles such as liposomes and microemulsions have also shown encouraging results (Ghanbarzadeh et al., 2015; Üstündağ Okur et al., 2019; Banihashemi et al., 2015) Solid lipid nanoparticles have been explored as attractive choices for topical delivery as they form an occlusive layer on the surface of the skin, leading to hydration of the stratum corneum and increased drug penetration. In addition, they offer many advantages such as high drug load, greater stability and bioavailability.
Liposomes
They are microscopic, spherical vesicles composed of a concentric phospholipid and cholesterol bilayer and can incorporate hydrophobic and hydrophilic drug. They can easily fuse with the cell membrane and change the fluidity of the membrane to effectively deliver the drug and enhance the penetration of the stratum corneum. Mi- croneedling has been suggested to enhance the active effects on the liposomal form.
Oral treatments
• Pentoxifylline 1,200 mg/day for 8 weeks: a hemorrheological agent that promotes microcirculation perfusion by improving blood flow and developing antithrombotic effects.
• Ascorbic acid 1,000 mg/day for a period of 8 months: it is an antioxidant radical scavenger and essential for collagen synthesis.
• Mixture of venoactive agents with oral flavonoids: Diosmin 450 mg, Hesperidin 50 mg and Euphorbia prostate extract 100 mg per day for 2 weeks. Decreases capillary permeability, inhibits the production of free oxygen radicals and lipid peroxidation, decreases prostaglandin and thromboxane synthesis, and decreases endothelial activation and blood viscosity.
• Calcium dobesilate 500 mg daily for 2 weeks: inhibition of prostaglandin and thromboxane synthesis, vascular relaxation due to nitrous oxide production, reduction of blood viscosity and down regulation of VEGF expression.
• Colchicine 0.5 mg for 8 weeks: immunomodulatory function due to blocking T-cell chemotaxis.
LIPOSOMAL LACTOFERRIN
Lactoferrin (LF) is an iron-binding glycoprotein with anti-inflammatory, antiviral, antibacterial, antifungal, antiparasitic and immunomodulatory properties. It has been investigated to treat various disorders. It has two highly homologous lobes with stable and reversible iron binding capacity. Therefore, it has chelating properties not only of iron ions, but also of copper and zinc, being used in situations of dermoepidermal dyschromia.
PREVENTION
Based on our clinical practice, I will now list some suggestions for preventing stains from controlling the hematoma that appears after the procedure.
1. Check the patient’s regular use medications: as previously mentioned, some medications may increase bleeding time or interfere with melanin production.
2. Control of subincision trauma: being as accurate as possible in cutting the septum of fibrosis greatly reduces adjacent trauma and, consequently, bruising.
3. Ideally compress the bleeding region for 5 to 10 minutes: this will reduce the bleeding time and consequently the bruising.
4. Ice packs – with care: ice in the immediate post-procedure and in the first days has an analgesic action, in addition to reducing edema and the formation of hematomas.
5. Through-the-orifice washing with ice-cold SF: same principle as using ice.
6. Compressive dressing – micropore/tape; keep it for 2-3 days: favors the reorganization of the scar tissue formed during the repair process. It also accelerates the resorption of ecchymotic areas and minimizes fluid accumulation.
7. Prescription Tranexamic Acid 250 mg – 2 cp. daily for 5-7 days: In one study, oral tranexamic acid was used off-label to successfully treat and/or prevent PIH in approximately 82 high-risk patients after injury or before procedures that disrupt the epidermis. TXA treatment can be used for all at-risk patients prophylactically before undergoing microneedling, cryotherapy, cryolipolysis, chemical peels, and laser treatments. However, it is contraindicated in patients with hypercoagulable conditions, renal failure, vision disorders, pregnancy, breastfeeding, or on hormonal therapies.
8. Use of the shorts for at least 7 days, ideally for 30 days: it helps to optimize the treatment, as the compressive force generated will contribute to the benefits of ecchymotic reabsorption and to minimize the accumulation of fluids.
9. Touch-up with more than 45 days of interval: by extending the return, the healing of the treated skin will be complete and the risk of hyperpigmentation is also reduced by not addressing a skin that is still in an inflammatory/cicatricial process.
10. If possible and feasible, guide the early use of a triple formula (tretinoin, corticoid and hydroquinone) or glycolic acid in patients who tend to hyperpigment.
11. Avoid sun exposure until the spots improve and use photoprotector
CONSIDERATIONS
Post-Goldincision® hyperpigmentation is a challenge because there is still no definitive and quick treatment. It is an alteration that can affect any phototype and that worries patients who want an effective solution and treatment of the problem. Because it is a multifactorial dyschromia, several depigmenting agents are used in order to combat this alteration, as well as the association with other treatments for a faster and more effective solution to the problem. I recommend that, whenever possible, the treatment be planned taking into account all the factors that can be controlled, in order to avoid hyperchromia.
REFERENCES
Abdel-Malek Z, Kadekaro A L, Swope VB. Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Research, v.23, p. 171– 186, 2010.
Abdel-Malek Z, Scott MC, Itaru S, Tada A, Im S, Lamoreux L, Ito S, Barsh G, Hearing VJ. The Melanocortin-1 Receptor is a Key Regulator of Human Cutaneous Pigmentation. Pigment Cell Research, v.13, supl. 8, p. 156-162, 2000.
ABIHPEC. Disponível em: <http://www.abihpec.org.br/conteudo/Panorama_do_setor_20092010_Portugues_12 _04_10.pdf >. Accessed on: 10.10.2010
Agne JE. Eu sei eletroterapia. Santa Maria: Pallotti, 2009.
Alam M, Gladstone HB, Tung RC. Deramatologia Cosmética. Rio de Janeiro: Elsevier, 2010.
Alappatt C, Johnson CA, Clay KL, Travers JB. Acute keratinocyte damage stimulates platelet-activating factor production. Archives of Dermatological Research, v. 292, n.5, p. 256-259, 2000.
Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Fundamentos da Biologia Celular. 2.ed., Porto Alegre: Artmed, 2006.
An SM, Koh JS, Boo YC. p-coumaric acid not only inhibits human tyrosinase activity in vitro but also melanogenesis in cells exposed to UVB. Phytoterapy Research, v.24, p. 1175 – 1180, 2010.
Ancans J, Tobin DJ, Hoogduijn MJ, Smit NP, Wakamatsu K, Thody AJ. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Experimental Cell Research, v. 268, p. 26–35, 2001.
Ando H, Kondoh H, Ichihashi M, Hearing VJ. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. Journal of Investigative Dermatology, v.127, p. 751-761, 2007.
Andrade MS, Sampaio TS, Nogueira PCL, Ribeiro AS, Bittrich V, Amaral MCE. Volatile compounds from leaves and flowers of Garcinia macrophylla. Chemistry of Natural Compounds, v. 43, no2, p. 221-224, 2007.
Arks MS, Seabra MC. The melanosome: membrane dynamics in black and white. Nature Reviews Molecular Cell Biology, v. 2, p. 738 – 748, 2001.
Azulay RD, Azulay, L. Dermatologia. 7.ed. São Paulo: Guanabara-Koogan, 2017.
Banihashemi M, Zabolinejad N, Jaafari MR, Salehi M, Jabari A. Comparação dos efeitos terapêuticos do ácido tranexâmico lipossomal e da hidroquinona convencional no melasma. J Cosmet Dermatol. 2015;14(3):174–177. doi: 10.1111/JOCD.12152.
Barbosa W, Chagas EA, Martins L, Pio R, Tucci MLS, Artiolo FA. Germinaçãção de sementes e desenvolvimento inicial de plântulas de achachairu. Revista Brasileira de Fruticultura, Jaboticabal – São Paulo, v.30, n.1, p.263-266, 2008.
Bernhard D, Schwaiger W, Crazzolara R, Tinhofer I, Kofler R, Csordas, A. Enhanced MTT-reducing activity under growth inhibition by resveratrol in CEM-C7H2 lymphocytic leukemia cells. Cancer Lett., v. 195, p. 193 – 199, 2003.
Berson JF, Harper DC, Tenza D, Raposo G, Marks MS. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol. Cell Biol., v.12, p. 3451–3464, 2001.
Bertolotto C, Buscà R, Abbe P, Bille K, Aberdam E, Ortone JP, Ballotti R. Different cis-Acting Elements Are Involved in the Regulation of TRP1 and TRP2 Promoter Activities by Cyclic AMP: Pivotal Role of M Boxes (GTCATGTGCT) and of Microphthalmia. Mol. Cell Biol., v. 18, p. 694 – 702, 1998.
Bhatia SK, Yetter AB. Correlation of visual cytotoxicity ratings of biomaterials with quantitative in vitro cell viability measurements. Cell Biology and Toxicology, v. 24, p. 315-319, 2008.
Bomfim VVBS et al. Peeling químico no tratamento de hipercromia pós inflamatória decorrente de acne. Research, Society and Development, v. 11, n. 7, e32611728745, 2022. DOI: http://dx.doi.org/10.33448/rsd-v11i7.28745.
Borges FS. Modalidades terapêuticas nas disfunçõções estéticas. 2.ed. São Paulo: Phorte, 2010.
Borkow G. et al. Improvement of facial skin characteristics using copper oxide containing pilloecases: a double-blind, placebo-controlled, parallel, randomized study.International Journal of Cosmetic Science, p. 1-7, 2009.
Botta B, Mac-Quhae MM, Delle Monache G, Delle Monache F, Demello JF. Chemical investigation of the genus Rheedia. V: Biflavonoids and xanthochymol. Journal of Natural Products, v. 47, p.1053, 1984.
Braz Filho R, Cavalcante de Magalhães G, Gottlieb OR. Xanthones of Rheedia gardneriana. Phytochemistry, v. 9,
p. 673, 1970.
Briganti S, Camera E, Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Research, v. 16, p. 101-110.
Buscà R, Ballotti R. Cyclic AMP a Key Messenger in the Regulation of Skin Pigmentation. Pigment Cell Research, v.13, p. 60 – 69, 2000.
Cameron M. Agentes físicos na reabilitação. 3 ed. Rio de Janeiro: Elsevier, 2009.
Campos PM. Avaliaçãção da atividade inibitória de melanogênese do extrato hidroalcoólico da Garcinia gardneriana (Planchon & Triana) Zappi. UFPR – Curitiba, 2019.
Castardo JA. Avaliaçãção da atividade do extrato hidroalcoólico bruto da Garcinia gardneriana (Planchon & Triana) Zappi em modelos experimentais de inflamaçãção aguda em camundongos. 136 f. Dissertaçãção (Mestrado em Farmacologia) – Setor de Ciências Biológicas, Universidade Federal do Paraná. Curitiba, 2007.
Castardo JA et al. Anti-inflammatory effects of hydroalcoholic extract and two biflavonoids from Garcinia gardneriana leaves in mouse paw oedema. Journal of Ethnopharmacology, Irlanda, v. 118, p. 405 – 411, 13 de agosto de 2008.
Catania AS et al. Vitaminas e minerais com propriedades antioxidantes e risco cardiometabólico: controversas e perspectivas. Arq Bras Endocrinol Metab. v. 3, p. 53-5, 2009.
Caymanchem. Available at: www.caymanchem.com. Accessed on 11.02.2010.
Cechinel Filho V et al. I3-naringenina-II8-4_-OMe-eriodictyol: a new potential analgesic agent isolated from Rheedia gardneriana leaves. Zeitschrift f ̈ ur Naturforschung, v. 55, p. 820–823, 2000.
Chang TS. An Updated Review of Tyrosinase Inhibitors. International Journal of Molecular Sciences, v.10, p. 2440-2475, 2009.
Chavantes MC. Laser em bio-medicina: princípios e prática: guia para iniciantes, pesquisadores e discentes na área da saúde e exatas. São Paulo: Atheneu, 2009.
Chawla S, Delong MA, Visscher MO, Wicket RR, Manga P, Boissy RE. Mechanism of tyrosinase inhibition by deoxyArbutin and its second- generation derivatives. British Journal of Dermatology, v. 159, p. 1267-1274, 2008.
Chemblink. Chemical Listing of Kojic acid. Available at: http://www.chemblink.com/products/501-30-4.htm. Accessed on 11.01.2010.
Chemistry. Available at: www.chemistry.about.com. Accessed on 11.02.2010.
Chen LG, Chang WL, Chia JL, Lee TL, Chwen MS, Wang CC. Melanogenesis inhibition by gallotannins from chinese galls in B16 mouse melanoma cells. Biological Pharmaceutical Bulletin, v. 32, p. 1447-1452, 2009.
Choi W, Miyamura Y, Wolber R, Smuda C, Reinhold HL, Kolbe L, Hearing VJ. Regulation of human skin pigmentation in situ by repetitive UV exposure: molecular characterization of responses to UVA and/or UVB. Journal of Investigative Dermatology, v.130, p. 1685-1696, 2010.
Chu D. Development and structure of skin. In: WOLF, K. et al. Fitzpatrick’s Dermatology in General Medicine. New York: McGraw-Hill, 2008, p. 57-73.
Costin, GE, Hearing, VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. Faseb J. 2007;2:976-94.
Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. The FASEB Journal, v. 21, p. 976-994, 2007.
Cymbalista NC, Garcia R, Bechara SJ. Classificação etiopatogênica de olheiras e preenchimento com ácido hialurônico: descrição de uma nova técnica utilizando cânula. Surgical Cosmetic Dermatology, v.4, n.4, p. 315-21, 2012.
Daguano JKMF, Santos C, Rogero SO. Avaliaçãção da citotoxicidade de biocerâmicas desenvolvida para uso em sistemas de implantes. Revista Matéria, v. 12, n. 11, p. 134-139, 2007.
Decker H, Schweikardt T, Tuczek F. The first crystal structure of tyrosinase: all questions answered? Angew Chem Int Ed Engl., v. 45, p. 4546-4550, 2006.
Delle Monache G et al. Minor xanthones from Rheedia gardneriana. Phytochemistry, v. 23, p. 1757-1759, 1984.
Delle Monache G, Botta B. Chemical investigation of the genus Rheedia, IV.Three new xanthones from Rheedia brasiliensis. Journal of Natural Products, v.47, p. 620-625, 1984.
Delle Monache G, Delle Monache F, Bettolo GBM. Chemical investigation of the genus Rheedia. II. Prenylated xanthones fromRheedia gardneriana. Journal of Natural Products, v. 46, p. 655-659, 1983.
DERMATOLOGIA. Available at: http://www.dermatologia.net/novo/base/atlas/melanoses_solares2.shtml http://www.dermatologia.net/novo/base/atlas/fotoenvelhec.shtml. Accessed on 11.01.2010.
Ding HY, Chou TH, Liang CH. Antioxidant and antimelanogenic properties of rosmarinic acid methyl ester from Origanum vulgare. Food Chemistry, v. 123, p. 254-262, 2010.
Doyle A, Griffiths JB. Cell and Tissue Culture: Laboratory Procedures in 1Biotechnology. John Wiley & Sons Ltd: Chichester, 1998.
Draelos ZD. Cosmecêuticos. 2.ed. Rio de Janeiro: Elsevier, 2009.
Draelos ZD. Skin lightening preparations and the hydroquinone controversy. Dermatologic Therapy, v. 20, p. 308-313, 2007.
Duval C, Smit NPM, Kolb AM, Re ́gnier M, Pavel S, Schmidt, R. Keratinocytes control the pheo/eumelanina ratio in cultured normal human melanocytes. Pigment Cell Research, UK, v.15, supl. 6, p. 440-446, 2002.
Eberlin S et al. Effects of a Brazilian herbal compound as a cosmetic eyecare for periorbital hyperchromia (‘‘dark circles’’). Journal of Cosmetic Dermatology. v. 8, p. 127-35, 2009.
Eller MS, Gilchrest BA. Tanning as part of the eukaryotic SOS response. Pigment Cell Research, v. 13, p. 248-252, 2000.
Elwing A, Sanches O. Drenagem linfática manual. São Paulo: Senac, 2010. Guirro E, Guirro R. Fisioterapia dermato-funcional: fundamentos, recursos, patologias. 3.ed. Barueri: Manole, 2010.
Espín JC, Wichers HJ. Effect of captopril on mushroom tyrosinase activity in vitro. Biochim. Biophys. Acta, v. 1544,
p. 289-300, 2001.
Fisher AA. Leukoderma from bleaching creams containing 2% hydroquinone. Contact Dermatitis, v. 8, p. 272–3, 1982.
Forslind B. The skin: upholder of physiological homeostasis. A physiological and (bio) physical study program. Thrombosis Research, v.80, n.1, p. 1-22, 1995.
Freeberg I. Keratinocytes. Available at:< http://www.aad.org/education/students/Keratinocytes.htm>. Accessed on: September 15, 2010.
Fuller BB, Spaulding DT, Smith DR. Regulation of the catalytic activity of preexisting tyrosinase in black and Caucasian human melanocyte cell cultures. Experimental Cell Research, v. 262, p. 197-208, 2001.
Garcia ES. Biodiversity, biotechnology and health. Cad. Saúde Públ., Rio de Janeiro, 11(3): 495-500, Jul/Sep, 1995.
Gartner LP, Hiatt JL. Tratado de Histologia. Rio de Janeiro: Editora Guanabara Koogan S.A., 2.ed., p 265-276, 2001.
Ghanbarzadeh S, Hariri R, Kouhsoltani M, Shokri J, Javadzadeh Y, Hamishehkar H. Maior estabilidade e entrega dérmica de hidroquinona usando nanopartículas lipídicas sólidas. Coloides Surf B Biointerfaces. 2015;136:1004–1010. doi: 10.1016/J.COLSURFB.2015.10.041.
Gilchrest BA, Park HY, Mark MS, Yaar M. Mechanism of ultraviolet light – induced pigmentation. Photochemistry and Photobiology, v. 63, n. 1, p. 1-10, 1996.
Goldberg D J. Laser em Dermatologia. São Paulo: Livraria Santos, 2007.
Gomes RK, Damazio MG. Cosmetologia: descomplicando os princípios ativos. 3.ed. São Paulo: Livraria Médica Paulista, 2009.
Gonchoroski DD, Correa GM. Tratamento de hipercromia pós-inflamatória com diferentes formulações clareadoras. Informa, v.17, n. 3/4, 2005.
Guimarães CL, Otuki MF, Beirith A, Cabrini DA. Uma revisão sobre o potencial terapêutico da Garci0nia gardneriana-NA. Dynamis Revista Tecno- Científica, v. 12, p. 6-12, 2004.
Haake A, Holbrook K. The structure and development of skin. In: Fitzpatrick TB et al. Fitzpatrick’s Dermatology in General Medicine. New York: McGraw-Hill, 1999, p. 70-114.
Hanamura T, Uchida E, Aoki H. Skin-lightening effect of a polyphenol extract from acerola (Malpighia emarginata DC) fruit on UV-induced pigmentation. Bioscience Biotechnological Biochemistry, v. 72, n. 12, p. 3211-3218, 2008.
Handog EB, FPDS, Datuin SL, Singzon IA. An open-label, single-arm trial of the safety and efficacy of a novel preparation of glutathione as a skin-lightening agent in Filipino women. International Journal of Dermatology, vol. 55, no. 2, p. 153-157, 2016.
Hearing VJ, Yamaguchi Y. Melanocyte distribution and function in human skin: Effects of ultraviolet radiation. In: Hearing VJ, Leong SPL. From Melanocytes to Melanoma: The Progression to Malignancy. New Jersey: Humana Press, 2006, p. 101-115.
Hearing VJ, Jimenez M. Mammalian tyrosinase – the critical regulatory control point in melanocyte pigmentation, Int. J. Biochem., v. 19, p. 1141-1147, 1987.
Hearing VJ. The regulation of melanin formation. In: NORDLUND, J.J. et al. The Pigmentary System: Physiology and Pathophysiology. Massachussets: Blackwell Publishing, 2006, 2a ediçãção, p. 191-212.
Hearing VJ, Tsukamoto K. Enzimatic control of pigmentation in mamals. The FASEB Journal. , v.5, p. 2902-2909, 1991.
Hernandez M, Fresnel MM. Manual de Cosmetologia. 3.ed. Rio de Janeiro: Revinter, 1999.
Horibe EK. Estética Clínica & Cirúrgica. Rio de Janeiro: Revinter, 2000. Jedwab SK. Laser e outras tecnologias na dermatologia. São Paulo: Santos, 2010.
Hosoi AJ, Suda KT. Regulation of melanin synthesis of B16 mouse melanoma cells by 1a,25-dihydroxyvitamin D3 and retinoic acid. Cancer Res., v. 45, p. 1474-1478, 1985.
Hu ZM, Zhou Q, Lei TC, Ding SF, Xu SZ. Effects of hydroquinone and its glucoside derivatives on melanogenesis and antioxidation: Biosafety as skin whitening agents. Journal of Dermatological Science, v. 55, p. 179-184, 2009.
Ito S, Wakamatsu K, Ozeki H. Chemical analysis of melanins and its application to the study of the regulation of melanogenesis. Pigment Cell Res., v.13, n. 8, p. 103-109, 2000.
Itoh K, Hirata N, Masuda M, Naruto S, Murata S, Wakabayashi K, Matsuda H. Inhibitory effects of Citrus hassaku extract and its flavanone glycosides on melanogenesis. Biol. Pharm. Bull., v. 32, no3, p. 410-415, 2009.
Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57-106.
Jedwab SKK. Laser e outras tecnologias na dermatologia. São Paulo: Santos, 2010.
Junqueira LC, Carneiro J, Abrahamsohn P. Histologia básica: texto e atlas. 13.ed. Rio de Janeiro: Guanabara Koogan, 2017.
Kalil CLPV. Laser e outras fontes de luz na dermatologia. Rio de Janeiro: Elsevier, 2011. Kede MPV, Sabatovich O. Dermatologia Estética. 2.ed. São Paulo: Atheneu, 2009.
Katsambas AD, Stratigos AJ. Depigmenting and Bleaching Agents: Coping with Hyperpigmentation. Clinics in Dermatology, v.19, p. 483-489, 2001.
Kessel, RG. Histologia Médica Básica: A biologia das células, tecidos e órgãos. Rio de Janeiro: Editora Guanabara Koogan, 2001, p. 318-331.
Kim DS, Jeong YM, Park IP, Hahn HG, Lee HK, Kwon SB, Jeong JH, Yang SJ, Sohn UD, Park KC. A new 2-imino-1,3-thiazoline derivative, KHG22394, inhibits melanin synthesis in mouse B16 melanoma cells. Biol. Pharm. Bull., v. 30, supl. 1, p. 180-183, 2007.
Kim DS, Park SH, Kwon SB, Joo YH, Youn SW, Sohn UD, Park KC. Temperature Regulates Melanin Synthesis in Melanocytes. Archives Pharmacal Research, v. 26, no 10, p. 840-845, 2003.
Kim YJ, No JK, Lee JS, Kim MS, Chung HY. Antimelanogenic activity of 3,4 – dihydroxyacetophenona: inhibition of tyrosinase and MITF. Bioscience Biotechnological Biochemistry, v. 70, p. 532-534, 2006.
Kim YJ, No JK, Lee JH, Chung HY. 4,4’- Dihydroxybiphenyl as a new potent tyrosinase inhibitor. Biol. Pharm. Bull., v.28, p. 323-327, 2005.
Kim YM, Yun J, Lee CK, Lee H, Min KR, Kim Y. Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J. Biol. Chem. v. 277, p. 16340-16344, 2002.
Klabunde T, Eicken C, Sacchettini JC, Krebs B. Crystal structure of a plant catechol oxidase containing a dicopper center. Nat. Struct. Biol., v. 5, p. 1084-1090, 1998.
Kushimoto T, Basrur V, Valencia JL, Matsunaga J, Vieira WD, Ferrans VJ, Muller J, Appella E, Hearing VJ. A model for melanosome biogenesis based on the purification and analysis of early melanosomes. Proc. Natl. Acad. Sci. U. S. A. v.98, p. 10698-10703, 2001.
Land EJ, Ramsdem CA, Riley PA, Stratford MR. Evidence consistent with the requirement of cresolase activity for suicide inactivation of tyrosinase. Tohoku J Exp Med, v . 216, p. 231–238, 2008.
Lapeere H et al. Hypomelanoses and hypermelanoses. In: Fitzpatrick, T. B. et al. Fitzpatrick’s Dermatology in General Medicine. New York: McGraw-Hill, p. 622- 640, 1999.
Le Mellay-Hamon V, Criton M. Phenylethylamide and Phenylmethylamide Derivatives as New Tyrosinase Inhibitors. Biological & Pharmaceutical Bulletin, v.32, n. 2, p. 301-303, 2009.
Leduc A, Leduc O. Drenagem linfática: teoria e prática, 3 ed. São Paulo: Manole, 2008.
Lee J, Jung E, Lee J, Huh S, Boo YC, Kim YS, Park D. Mechanism of melanogenesis inhibition by 2,5-dimethyl-4-hydroxy-3 (2H)-furanone. British Journal of Dermatology, v. 157, p. 242-248, 2007.
Lee MY, Kim JH, Choi JN, Kim J, Hwang GS, Lee CH. The melanin synthesis inhibition and radical scavenging activities of compounds isolated from the aerial part of Lespedeza cyrtobotrya. Journal of Microbiology and Biotechnology, v.20, no. 6, p. 988-994, 2010.
Lee YS, Kim HK, Lee KJ, Jeon HW, Cui S, Lee YM, Moon BJ, Kim YH. Inhibitory effect of glyceollin isolated from soybean against melanogenesis in B16 melanoma cells. BMB reports., v. 43, p. 461-467, 2010.
Lehninger AL, Nelson DL, Cox MM. Lehninger Princípios de Bioquímica. 4.ed., São Paulo: Ed. Sarvier, 2006.
Li XC, Joshi AS, Tan B, Ehsohly HN, Walker LA, Zjawiony JK, Ferreira D. Absolute configuration, conformation, and chiral properties of flavanone-(3→8”)-flavone biflavonoids from Rheedia acuminata. Tetrahedrom, v. 58, p. 8709-8717, 2002.
Li X, Guo L, Sun Y, Zhou J, Gu Y, Li Y. Baicalein inhibits melanogenesis through activation of the ERK signaling pathway. International Journal of Molecular Medicine, v. 25, p. 923-927, 2010.
Lim JT. Treatment of melasma using kojic acid in a gel containing hydroquinone and glycolic acid. Dermatologic Surgery, v. 25, no4, p. 282-284, 1999.
Lim YJ, Lee EH, Kang TH, Ha SK, Oh MS, Kim SM, Yoon TJ, Kang C, Park JH, Kim SY. Inhibitory effects of arbutin on melanin biosynthesis of α- melanocyte stimulating hormone – induced hyperpigmentation in cultured brownish guinea pig skin tussues. Archives of Pharmacal Research, v. 32, n. 3, p. 367-373, 2009.
Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007; 445:843-50.
Lin HC, Shieh BH, Lu MH, Chen JY, Chang LT, Chao CF. A method for quantifying melanosome transfer efficacy from melanocytes to keratinocytes in vitro. Pigment Cell Melanoma Research, v. 21, p. 559-564, 2008.
López-Machado A, Díaz-Garrido N, Cano A, Espina M, Badia J, Baldomà L, Calpena AC, Souto EB, García ML, Sánchez-López E. Development of Lactoferrin-Loaded Liposomes for the Management of Dry Eye Disease and Ocular Inflammation. Pharmaceutics. 2021 Oct 15;13(10):1698. doi: 10.3390/pharmaceutics13101698. PMID: 34683990; PMCID: PMC8539938.
Luvizon AC. Modulaçãção fenotípica induzida por guanosina em modelo de melanoma murino (B16F10). 83 f. Dissertaçãção (Mestrado em Microbiologia).
Luzzi R, Guimaraes CL, Verdi LG, Simionatto EL, Delle Monache F, Yunes RA, Floriani AEO, Cechinel Filho V. Isolation of biflavonoids with analgesic activity from Rheedia gardneriana leaves. Phytomedicine, v. 4, p. 139-142, 1997.
Maeda K, Naitou T, Umishio K, Fukuhara T, Motoyama A. A novel melanin inhibitor: hidroperoxy traxastane-type triterpe0ne from flowers of Arnica Montana. Biological & Pharmaceutical Bulletin, v. 30, supl. 5, p. 873-879, 2007.
Maeda K, Fukuda M. Arbutin: mechanism of its depigmenting action in human melanocyte culture. The Journal of Pharmacology and Experimental Therapeutics. v. 276, supl. 2, p. 765-769, 1996.
Maeda K, Fukuda M. In vitro effectiveness of several whitening cosmetic components in human melanocytes. Journal of the society of cosmetics scientists, v. 42, p. 361-368, 1991.
Marcio G. Mecanismo de açãção dos Despigmentantes. https://marcioguidoni.com.br/cosmetologia/mecanismo-de-ação-dos-despigmentantes/
Martinez M, Rittes P. Beleza sem Cirurgia. Tudo que você pode fazer para adiar a plástica. 2.ed. São Paulo: Senac, 2003.
Masuda T et al. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitor from Garcinia subelliptica. Bioscience, Biotechnology, and Biochemistry, Japão, v.69, p. 197-201, 2005.
Merchant D, Kahn R, Murphy W. Handbook of cell and organ culture. Burgess Publishing, Broken Arrow. 1964.
Miot LDB, Miot HA, Silva MG, Marques MEA. Estudo comparativo morfofuncional de melanócitos em lesőes de melasma. An Bras Dermatol. 2007;82:529-64.
Miot, LDB et al. Fisiopatologia do melasma. Anais Brasileiros de Dermatologia [online]. 2009, v. 84, n. 6 [Acessado 7 Julho 2022], p. 623-635. Available at: <https://doi.org/10.1590/S0365-05962009000600008>. Epub 25 Fev 2010. ISSN 1806-4841. https://doi.org/10.1590/S036505962009000600008.
Miyazaki SF. Utilização do chá-verde em cosmética. Cadernos de Prospecção. v. 1, n. 1, p. 10-3, 2008. NUNO, O. Laser em dermatologia: conceitos básicos e aplicações. 2.ed. São Paulo: Roca, 2009.
Miyazawa M, Tamura N. Inhibitory Compound of Tyrosinase Activity from the Sprout of Polygonum hydropiper L.
(Benitade). Biological & Pharmaceutical Bulletin, v. 30, n. 3, p. 595-597, 2007.
Momtaz S, Mapunya BM, Houghton PJ, Edgerly C, Hussein A, Naidoo S, Lall N. Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. Journal of Ethnopharmacology, v.119, p. 507-512, 2008.
Morimura K, Hiramatsu K, Yamazaki C, Hattori Y, Makabe H, Hirota M. Daedalin A, a metabolite of Daedalea dickinsii, inhibits melanin synthesis in an in vitro human skin model. Bioscience Biotechnology Biochemistry, v. 73, n. 3, p. 627-632, 2009.
Mosher DB, Fitzpatrick TB, Ortonne JP, Hori Y. Normal skin color and General Considerations of Pigmentary Disorders. In: Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF. Dermatology in General Medicine. v. 1. New York: Mcgraw-Hill; 1999. p. 936-44.
Nakagawa M, Kawai K, Kawai K. Contact allergy to kojic acid in skin care products. Contact Dermatitis, v.32, p.9-13, 1995.
Nakajima M, Shinoda I, Fukuwatari Y, Hayasawa H. Arbutin increases the pigmentation of cultured human melanocytes through mechanism other than the induction of tyrosinase activity. Pigment Cell Res., v.11, p. 12-17, 1998.
Nappi AJ, Vass E. Hydrogen peroxide generation associated with the oxidations of the eumelanogennic precursors 5,6 – dihydroxyindole and 5,6 – dihydroxyindole-2- carboxylic acid. Melanona Research, v. 6, p. 341-349, 1996.
Narayanan DL, Saladi RN, Fox J. L.,Ultraviolet radiation and skin cancer. International Journal of Dermatology, v. 49, 978–986, 2010.
Nesterov A, Zhao J, Minter D, Hertel C, Ma W, Abeysinghe P, Hong M, Jia Q. 1-(2,4-Dihydroxiphenyl)-3-(2,4-dimethoxy-3- methylphenyl)propane, a novel tyrosinase inhibitor with strong depigmenting effects. Chemical Pharmaceutical Bulletin, v. 56, supl. 9, p. 1292-1296, 2008.
Nicoletti MA, Orsine EMA, Duarte ACN, Buono GA. Hipercromias: aspectos gerais e uso de despigmentantes cutâneos. Cosmetics & Toiletries (ediçãção em português), São Paulo, v. 14, p. 46-51, mai-jun/2002.
Obolskiy D, Pischel I, Siriwatanametanon N, Heinrich M. Garcinia mangostana L.: A Phytochemical and Pharmacological Review. Phytotherapy Research, v. 23, p. 1047-1065, 2009.
Ohshima H et al. Effects of vitamin C on dark circles of the lower eyelids: quantitative evaluation using image analysis and echogram. Skin Research and echnology. v. 15, p. 214.
Ohshima H, Takiwaki H. Evaluation of dark circles of the lower eyelid: comparison between reflectance meters and image processing and involvement of dermal thickness in appearance. Skin Research and Technology. v. 14, p. 135-41, 2008.
Okunji C, Komarnytsky S, Fear G, Poulev A, Ribnicky DM, Awachie PI, Ito Y, Raskin I. Preparative isolation and identification of tyrosinase inhibitors from the seeds of Garcinia kola by high-speed counter-current chromatography. Journal of Chromathography A, v. 1151, supl. 1-2, p.45-50, 2007.
Ortonne JP [homepage on the Internet]. Skin color variations in humankind: an explanation? Nice: Pigmentary Disorders Academy; 2005 [cited 2009 Jun 23]. Available from: http://www.pigmentarydisordersacademy.org/guest_editorials_ortonne_skincolorjsp.
Ortonne JP, Bissett DL. Latest insights into skin hyperpigmentation. Journal of Investigative Dermatology Symposium Proceedings, v. 13, p. 10-14, 2008.
Pandya AG, Guevara IL. Disorders of Hyperpigmentation. Dermatologic Clinics. Dallas, Texas, v.18, supl.1, p. 91-98, jan/2000.
Park HY, Kosmadaki M, Yaar M, Gilchrest BA. Cellular mechanism regulating human melanogenesis. Cellular and Molecular Life Sciences, Birkhäuser, vol. 66, no. 9, p. 1493-1506, 2009.
Park HY, Pongpudpunth M, Lee J, Yaar M. Disorders of melanocytes. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ. Fitzpatrick’s Dermatology in General Medicine. 7.ed. New York: Mc Graw Hill Medical, p. 591-608, 2008.
Parolin MB, Reason IJM. Apoptose como mecanismo de lesão nas doenças hepatobiliares. Arquivos Gastroenterologia, v. 38, n. 2, p. 138-144, 2001.
Parvez S, Kang M, Chung HS, Bae H. Naturally occurring tyrosinase inhibitors: mechanism and applications in skin health, cosmetics and agriculture industries. Phytotherapy Research, v. 21, p. 805-816.
Patologia: Processos Gerais – Andrade Netto Brito Montenegro. Editora Atheneu. 3.ed.
Perluigi M, De Marco F, Foppoli C, Coccia R, Blarzino C, Marcante ML, Cini C. Tyrosinase protects human melanocytes from ROS – generating compounds. Biochemical and Biophysical Research Communication, v. 305, p. 250-256, 2003.
Pinto AC, Silva DHS, Bolzani VS, Lopes PN, Epifanio RA. Produtos naturais: atualidade, desafios e perspectivas. Química Nova, São Paulo, v.25, supl.1, p.45-61, 2002.
Prota G. Recent Advances in the Chemistry of Melanogenesis in Mammals. The Journal of Investigative Dermatology, v. 75, p. 122-127, 1980.
Quezada GN, Romero, W. Dermatoscopia na hiperpigmentaçãção periorbital: uma ajuda no diagnóstico do tipo clínico. Artigo capturado às 20h23min do dia 19/04/2015 de http://portalbiocursos.com.br/ohs
Reid K, Nishikawa S, Barlett PF, Murphy M. Steel factor directs melanocyte development in vitro through selective regulation of the number of c-kit+ progenitors. Developmental biology, v.169, p. 568–579, 1995.
Relethford JH. Hemispheric difference in human skin color. Am J Phys Anthropol.1997;104:449-57.
Ribeiro CJ. Cosmetologia aplicada a dermocosmética, 2 ed. São Paulo: Pharmabook, 2010.
Rodrigues CA, Oliveira AE, Willain FR, Cechinel Filho CL, Guimaraes CL, Yunes RA, Delle Monache F. Separation of biflafonoids from Rheedia gardneriana using chitin-Fe complex as stationary phase. Pharmazie, v. 55, p. 699-700, 2000.
Roh MR, Chung KY. Infraorbital Dark Circles: Definition, Causes, and Treatment Options. Dermatol Surg v. 35, n.2, p. 1163-1171, 2009.
Roméro C, Aberdam E, Larnier C, Ortonne JP. Retinoic acid as modulator of UVB-induced melanocyte differentiation involvement of the melanogenic enzymes expression. Journal of Cell Science, v. 107, p. 1095-1103, 1994.
Rumjanek VM, Trindade GS, Wagner-Souza K, De-Oliveira MC, Marques-Santos LF, Maia RC, Capella MA. Multidrug resistance in tumour cells: characterization of the multidrug resistant cell line K562-Lucena 1. Anais da Academia Brasileira de Ciências, v.73, n.1, p.57-69. 2001.
Sampaio S, Rivitti EA. Dermatologia. 3.ed. São Paulo: Artes Médicas, 2008. Trajano RW. Laserterapia: manual LED ultra blue. DMC. São Paulo, 2011.
Sampaio SAP, Rivitti EA. Dermatologia. 4.ed. Porto Alegre: Artes Médicas, 2018.
Sato K, Morita M, Ichikawa C, Takahashi H, Toriyama M. Depigmenting mechanisms of all-trans retinoic acid and retinol on B16 melanoma cells. Bioscience Biotechnology Biochemistry, v. 72, supl. 10, p. 2589-2597, 2008.
Sato K, Takahashi H, Iraha R, Toriyama M. Down-regulation of tyrosinase expression by acetylsalicylic acid in murine B16 melanoma. Biological & Pharmaceutical Bulletin, v.31, supl. 1, p. 33-37, 2007.
Schroterova L, Kralova V, Voracova A, Haskova P, Rudolf E, Cervinka M. Antiproliferative effects of selenium compounds in colon cancer cells: Comparison of different cytotoxicity assays. Toxicology in vitro, v. 23, p. 1406-1411, 2009.
Schwahn DJ, Xu W, Herrin AB, Bales ES, Medrano EE. Tyrosine levels regulate the melanogenic response to α – melanocyte – stimula0ting hormone in human melanocytes: implications for pigmentation and proliferation. Pigment Cell Research, v. 14, p. 32-39, 2001.
Seiji M, Shimao K, Birbeck, MS, Fitzpatrick TB. Subcellular localization of melanin biosynthesis. Annals of New York Academy Science, v.100, p. 497-533, 1963.
Serra-Baldrich E, Tribó MJ, Camarasa JG. Allergic contact dermatitis from kojic acid. Contact Dermatitis, v. 39, no 2, p. 86-87, 1998.
Shin NH, Ryu SY, Choi EJ, Kang SH, Chang IM, Min KR, Kim Y. Oxyresveratrol as the potent inhibitor on dopa oxidase activity of mushroom tyrosinase. Biochem. Biophys. Res. Commun., v. 243, p. 801-803, 1998.
Silva EC. Desenvolvimento e avaliaçãção da estabilidade de formulaçõções contendo arbutina, associada ou não com ácido glicólico, 181 f., (Tese de doutorado – Faculdade de Ciências Farmacêuticas – USP), São Paulo, 1998.
Silva TMA, Aoyama H, Haun M, Ferreira CV. Citotoxicidade do promotor de tumor e sua açãção mitogênica sobre os linfócitos humanos. Revista Brasileira de Análises Clínicas, v. 36(4), p. 237-239, 2004.
Simões CMO, Schenkel EP. A pesquisa e a produçãção brasileira de medicamentos a partir de plantas medicinais: a necessária interaçãção da indústria com a academia. Revista Brasileira de Farmacognosia, João Pessoa, v. 12, n.1, p. 35-40, 2002.
Slominski A, Desmond JT, Shigeki S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiological reviews. v. 84, p. 1155-1228, 2004.
Solano F, Briganti S, Picardo M, Ghanem G. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Research, v. 19, supl. 6, p. 550-571, 2006.
Spellberg B. The cutaneous citadel: a holistic view of skin and immunity. Life Sciences, v. 67, p. 477-502, 2000.
Spigariolo CB, Giacalone S, Nazzaro G. Dermatoses Púricos Pigmentados: Uma Revisão Narrativa Completa. J Clin Med. 25 de maio de 2021; 10(11):2283. doi: 10.3390/jcm10112283. PMID: 34070260; PMCID: PMC8197337.
Suh KS, Baek JW, Kim TK, Lee JW. The inhibitory effect of phytoclear- EL1 on melanogenesis. Annals of Dermatology, v. 2, n. 4, p. 369-375, 2009.
Sulaimon SS, Kitchell BE. The biology of melanocytes. Vet Dermatol. 2003;14: 57-65.
Sulaimon SS, Kitchell BE. The biology of melanocytes. Veterinary Dermatology, v. 14, p. 57-65, 2003.
Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Magnusson KP, et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39:1443-52.
Thompson JE. A prática farmacêutica na manipulaçãção de medicamentos. 1.ed. Porto Alegre: Artmed, 2006.
TRADE TERMS. Available at: <http://www.amlinkint.com/English/resources/trade- term-exw.html>. Accessed on: 07/21/2009.
Turk B, Turk V. Lysosomes as “suicide bags” in cell death: myth or reality? The Journal of Biological Chemistry,
v. 284, n.33, p. 21783-21787, 2009.
Üstündağ Okur N, Çağlar EŞ, Pekcan AN, Okur ME, Ayla Ş. Preparação, otimização e avaliação anti-inflamatória in vivo de formulações de microemulsão carregadas com hidroquinona para tratamento de melasma. J Res Pharm. 2019;23(4):662–670. doi: 10.12991/JRP.2019.174.
Van den Berg ME. Revisão das espécies brasileiras do gênero Rheedia L. (Guttiferae). Acta Amazônica, Manaus. v.9, n.1, p.43-74. 1979.
Verdia LG, Pizzolattia MG, Montanhera ABP, Brighentea IMC, Smania Jr A, Smania EFA, Simionatto EL, Monache FD. Antibacterial and brine shrimp lethality tests of biflavonoids and derivatives of Rheedia gardneriana. Fitoterapia, v. 75, p. 360-363, 2004.
Virador VM, Kobayashi N, Matsunaga J, Hearing VJ. A standardized protocol for assessing regulators of pigmentation. Analytical Biochesmistry, v. 270, p. 207-219, 1999.
Weiner H. Enzimas: classificaçãção, cinética e controle. In: Devlin TM et al. Manual de bioquímica com correlaçõções clínicas. São Paulo: Edgar Blucher, 2007, p. 374 – 391.
Wikipedia. Available at: http: www.pt.wikipedia.org/wiki/Garcinia_gardneriana. Accessed on: 11.04.2010.
Wolk K, Witte K, Sabat R. Interleukin-28 and Interleukin-29: Novel Regulators of Skin Biology. Journal of Interferon & Cytokine Research, v.30, no 8, p. 617-628, 2010.
Worfel PR. Análise da viabilidade e níveis de glutationa de células de melanoma murino tratadas com flavonóides e oxigênio singlete. 86 f. Dissertaçãção (Mestrado em Ciências – Bioquímica) – Setor de Ciências Biológicas, Universidade Federal do Paraná. Curitiba, 2009.
Yang H, Figueroa M, To S, Baggett S, Jiang B, Basile MJ, Weinstein BI, Kennelly EJ. Benzophenones and biflavonoids from Garcinia livingstonei fruits. Journal of Agricultural and Food Chemistry, v.26, 2010.
Yokota T, Nishio H, Kubota Y, Mizoguchi MM. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Research, v. 11, p. 355-361, 1998.
Zhang X, Hu X, Hou A, Wang, H. Inhibitory effect of 2,4,2’,4’-tetrahydroxy-3- (3-methyl-2-butenyl)- chalcone on tyrosinase activity and melanin biosynthesis. Biological Pharmaceutical Bulletin. v.32, no1, p. 86-90, 2009.
Zuidhoff HW, Rijsbergen JM van. Whitening efficacy of frequently used whitening ingredients. Cosmetics & Toiletries, v. 116, no1, p. 53-59, 2001.
