PMMA – Polymethylmethacrylate
Polymethyl methacrylate has been used in medicine for over 70 years. Its applications are diverse, such as bone cement composition, intraocular lens, tooth resin, skullcap closure material, and many others.
Today, PMMA has been an option in lipodystrophy filling – caused in HIV-positive patients – facial filling, chin bioplasty, cheekbone bioplasty, mandibular contour bioplasty, lip bioplasty, frontal grooves nasolabial (Chinese mustache) and labiogenic furrow, wrinkle bioplasty and scar repair bioplasty.
In addition, it allows its use for body filling, both aesthetically and reconstructively, as well as traumatized patients, patients with polio (infantile paralysis), patients with total or partial pectoral agenesis (Poland Syndrome), buttocks harmonization and correction of irregularities and depressions.
The use of filler substances has become popular in medicine, either for aesthetic purposes, making a face or body contour more attractive to the eye of the eye, or performing the necessary correction of irregularities arising with aging.
For both cases, we often need a correction with filling, as the asymmetry caused by aging, or the filling required for a good mandibular contour, or chin and malar projection, or in the patient with HIV + lipodystrophy, Parry’s Syndrome Romberg (progressive partial hemiatrophy), or body harmonization, gluteus correction, Poland syndrome, poliomyelitis sequelae, congenital clubfoot, among others. For these, a filling with liquid implant is required. This prevents us from making a significant difference with short-lived products of 1 or 2 years, making it necessary to use a longer lasting or permanent product like PMMA.
O PMMA (único implante permanente liberado pela ANVISA), a hidroxiapatita de cálcio (duração de um ano e meio), e o ácido polilático (duração de um ano e meio), embora sejam implantados no paciente sob a forma líquida, o produto na sua essência é sólido e todos atuam promovendo estimulação tecidual com neocolagênese, como veremos a seguir. O resultado final destes implantes permanecem sólidos, às custas de 20% implante injetável e 80% reação tecidual com neocolagênese, impossibilitando migração. São produtos menos elásticos, mais densos, onde precisamos cuidar com acúmulos e regiões superficiais, não sendo a melhor opção em naturalidade para preenchimento de lábios, por exemplo.
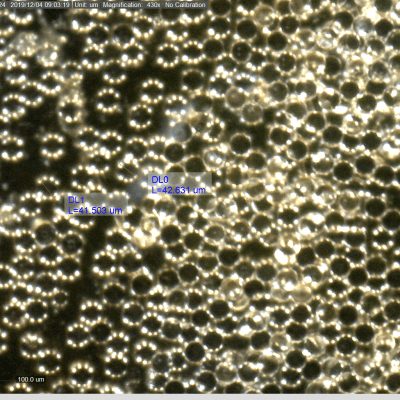
O PMMA com concentrações entre 2 a 10%, 20 a 30%, a hidroxiapatita com 30% e o Sculptra, que varia conforme diluição, na análise microscópica, todos possuem partículas circulares, sólidas, com superfície lisa e controle no diâmetro, que necessariamente precisa possuir um padrão de aproximadamente 40 micras de diâmetro. Isso porque partículas menores que 30 podem ser fagocitadas e favorecerem complicações clínicas, bem como partículas maiores que 50 micras não promovem o mesma reação tecidual e benefício em estimulação de colágeno. Além de não permitirem uso de microcânulas de pequeno calibre e favorecerem, juntamente com a irregularidade no tamanho e padrão das partículas, o aparecimento de granuloma (principal complicação no uso de PMMA de má qualidade) conforme já amplamente estudado e publicado por Lemperle.
Atualmente, apenas uma das três indústrias fabricantes de PMMA do Brasil produzem o produto com os pré-requisitos de partículas homogêneas, com tamanho controlado conforme microscopia enviada ao Congresso Europeu de Dermatologia por Dr. Chacur e apresentado em Outubro de 2016, na Áustria.
Several filler products are released in Brazil, some in gel form, which remain gel while present in the body, such as dozens of hyaluronic acids (lasting from six months to one and a half months), Polyethylene Glycol (trade name Remake), lasting approximately two years or more.
We had available the 3- to 6-year Aqualift (hydrogel 96 – 98% water with 2-4% polyamide), which expired in March 2014, and also the dreaded DMS (dimethylsiloxane) or liquid silicone, sometimes without purification, which is not registered with ANVISA, but even today has been used mainly by non-doctors in large quantities, usually in the gluteus. This is usually applied by beauticians or nursing technicians in environments without working permits or health surveillance (often in hotels or aesthetics). All of these products are applied in gel form and remain so during their presence in the body. As every procedure has risks, one of them, especially when applied in large quantities is migration, which does not occur with 1 ml on lips or fine wrinkles, but can occur in large quantities.
PMMA (the only permanent implant released by ANVISA), calcium hydroxyapatite (duration of one and a half years), and polylactic acid (duration of one and a half years), although they are implanted in the patient in liquid form, the product in its essence is solid and all work promoting tissue stimulation with neocolagenesis, as we will see below. The end result of these implants remains solid, at the expense of 20% injectable implant and 80% tissue reaction with neocolagenesis, making migration impossible. They are less elastic, denser products, where we need to take care of accumulations and superficial regions, not being the best natural option for lip filling, for example.
PMMA with concentrations of 2 to 10%, 20 to 30%, hydroxyapatite with 30% and Sculptra, which varies by dilution, on microscopic analysis all have solid circular particles with a smooth surface and diameter control, which necessarily it must have a standard of approximately 40 microns in diameter. This is because particles smaller than 30 can be phagocytized and favor clinical complications, as well as particles larger than 50 microns do not promote the same tissue reaction and benefit in collagen stimulation. In addition to not allowing the use of small-caliber microcannulas, they favor, together with the irregularity in particle size and pattern, the appearance of granuloma (main complication in the use of poor quality PMMA) as already widely studied and published by Lemperle.
Currently, only one of the three PMMA manufacturing industries in Brazil produces the product with the homogeneous particle size controlled requirements under microscopy sent to the European Congress of Dermatology by Dr. Chacur and presented in October 2016 in Austria.
About Polymethyl Methacrylate
Polymethyl methacrylate was developed in 1902 by the German chemist Rohn, was patented between 1928 and 1933. It was first used in 1940, being used in 1947 as a rib prosthesis by Judet, and in 1960 as a bone cement by Charnley. In 1985, animal studies as a liquid implant, in 1989 first human clinical studies (Frankfurt), and in 2003 the first publications by Lemperle.
Used for more than 76 years in the human body, PMMA is still widely used in orthopedics, dentistry, ophthalmology and aesthetics. We cannot deny the excellent biocompatibility with the body, where we have no allergic reaction or rejection to this product. Unlike products with “gels” end results, PMMA does not infect late, as the tissue result does not serve as a bacterial culture medium, as we had in other products.
Tissue Reaction
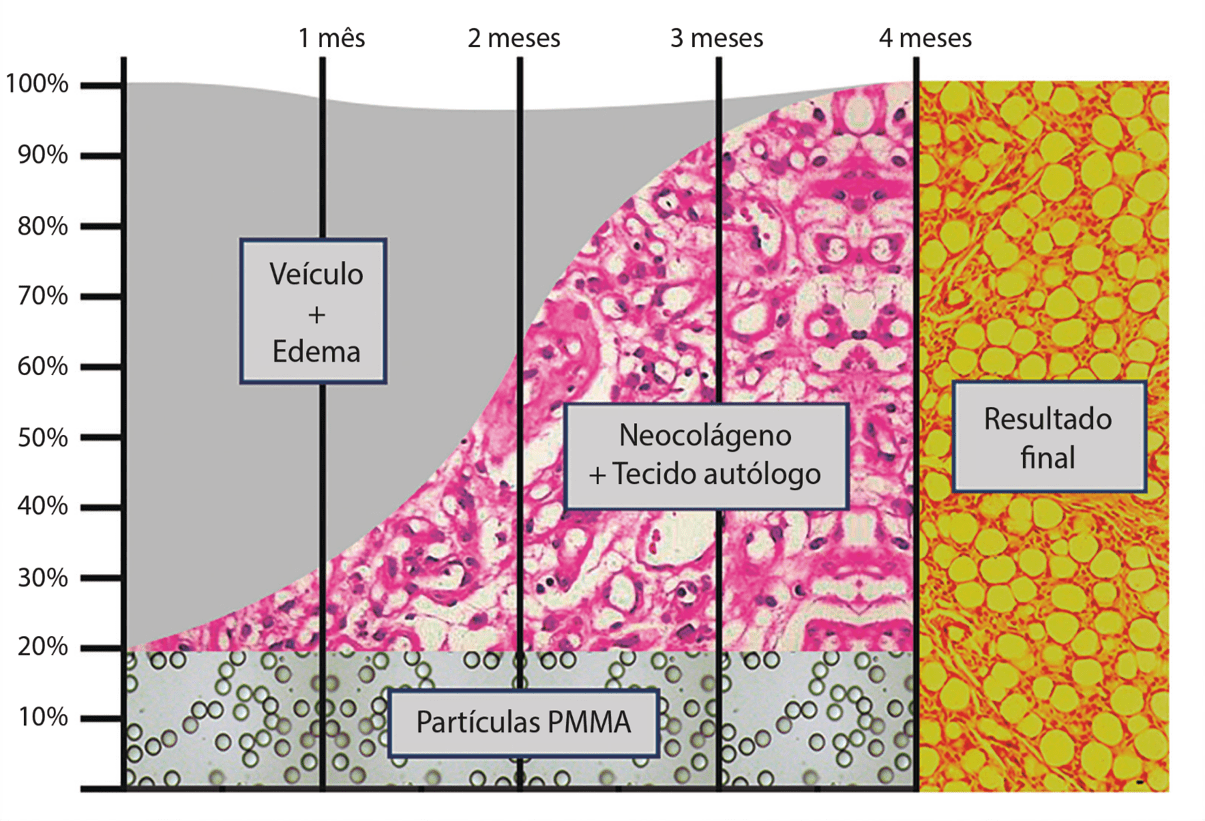
Marketed in various concentrations, two of the three ANVISA manufacturers in Brazil (also released by the FDA at a concentration of 20% since 2006) use carboxymethylcellulose as a vehicle, and one uses hydroxymethylcellulose. This vehicle is absorbed gradually between seven and 21 days and, parallel to this, a tissue reaction forms around each of the particles in the products, resulting in connective tissue, where the result of the product becomes solid and rich in collagen. .
Trying to quantify this reaction, which depends a lot on the product (microparticle quality), with the ideal product, 20% particles with 40 microns in diameter, we will have five million microprotheses per ml of injected product. These five million particles are responsible for stimulating 80% of their amount in the patient’s own tissue, that is, the PMMA implant works more as the patient’s own tissue stimulator, as properly filler.
As practiced by these authors, this stimulus may vary slightly among clinically treated patients. As well as healing, it is believed that black patients may have greater tissue gain in deeper regions, such as bone fair or intramuscular, the inflammatory process is smaller and consequently less tissue stimulation.
RESULTS OF PMMA TREATMENTS
Association of pan facial filling with 30 ml of PMMA in several planes and concentrations, being predominantly just periosteum, correcting the loss of age tissue. Improving (not just with padding, but with neocolagenesis) muscle laxity by submuscular padding. The use of fractionated CO2 laser and botulinum toxin as a complementary procedure was also associated in this case.
Before and after 14 days of evolution. Only one day with combined treatment between PMMA facial pan filling, approximately 20 ml of product between malar, nasojugal groove, nasogenian and labiogenic grooves, mandibular contour, labial and temporal contour, and fractional CO2 laser and botulinum toxin at the same time. .
As with other particulate products, the result of PMMA is not limited to filling, but moreover, it is permanent (as well as the loss of aging tissue) promoting a significant gain in skin quality through neocolagenesis.
Buttock filling with PMMA, performed in the office under local anesthesia, a post that requires only 7 days of physical activity. A very satisfactory and natural result (one of the main complaints of patients who implant silicone implants).
Another result of glute matching demonstrating not only the gain in shape, but the quality of the skin associated with subcision.
PMMA-treated polio sequelae: an excellent result that restored the patient’s self-esteem. A difficult or unsolvable treatment with another technique was performed in three steps with 100 ml of PMMA on each treatment day, with a minimum interval of 30 days between sessions.
Better facial harmony and more attractiveness with mandibular angle fill, malar arch and chin detail.
The results obtained above were only possible with a permanent product, since with the filling, the modifications are very visible not only in the harmonization, but in the skin quality due to the neocolagenesis.
RISKS ON USING PMMA
As well as the risk of any infiltrating fluid implant, the most feared risk is vascular obstruction, which is preventable or minimized with the use of atraumatic microcannulas, back-injection and slowly infiltration. When a vascular obstruction problem occurs soon after application, the occurrence of necrosis reported in some studies or even ophthalmological occurrence, with visual impairment, was observed soon after the procedure with evolution, in the first days, and could occur with any one. implants and not just PMMA.
Risks such as migration in the case of patients coming to the office from other professionals, none were using Polymethyl Methacrylate (PMMA).
Rejection or product allergy were not observed in the clinical practice of these two authors using PMMA, as well as late infection.
The real risks with PMMA use were granuloma (although very rare, but some cases related to poor quality products were observed).
Prior to 1994, particles of the product called Arteplast did not have adequate particle size control, and the risk of granuloma was estimated at 2.5%. In 1994, with proper particle size control, the risk of granuloma dropped to less than 0.01%, according to a study published by Lemperle in 1996. Artecoll release was granted by the EC, and only after the purification of Artecoll that In October 2006, PMMA was released in the US under the name Artefill (currently under the trade name Bellafill).
A very great care to be taken with the use of PMMA is inherent in the result, which needs to be good, because it is a difficult product to remove. We advise a lot of practice with fillers, as well as the professional must feel very confident of the indication and the result, which needs to be gradual, in front of a mirror, so that the patient can follow the procedure, which in 30 days will be reviewed and complemented. as needed.
Accumulation of product in surface regions should be avoided to prevent lumps from appearing as, as reported, the end result of applying PMMA is an autologous solid tissue rich in collagen but solid and more consistent than fat.
DISCUSSION
PMMA has always been widely used in medical and aesthetic practice, always very well accepted and tolerable by the body.
The results achieved are very satisfactory and depend a lot on the skill of each professional.
For some patients who undergo the procedure with unqualified professionals, usually non-medical, PMMA was stigmatized in the medical society, very poorly spoken in plastic surgery and dermatology.
Today there is no experienced doctor who used quality methacrylate, with the right practice, who stopped using the product due to patient problems. What is there is a doctor who has never used the product and, today, speaks badly due to patients looking for them with problems regarding the “product”, which most often is not polymethyl methacrylate. In general, they are patients who used “hydrogels” not registered with ANVISA and applied by untrained professionals.
CONCLUSION
The fact that we need a permanent product to achieve natural rejuvenation, made us improve the PMMA, already widely used in medicine and considered as an inert product, moldable at the time of application and that does not migrate or infect late.
The authors work exclusively with fillers and are the main “inspectors” of the products to be used, so both use the product with controlled particles, which may justify the very low incidence of complications.
Prior to 2007, Brazilian-handling pharmacies were free to produce PMMA, however the quality was very poor and none of the products met the particle quality needs. Today, the three products on the market are concerned with quality, two of which are in the final stages of improvement. It is believed that by the end of 2016 we will have all standard particle size products, which will further minimize the risk of using this already very low product.
BIBLIOGRAPHIC REFERENCES:
1 – Chacur R, Histological analysis of tissue reaction to polymethyl methacrylate used in musculature and subcutaneous tissue in Wistar rats. Work completion residency Surgery – Canoas 2008.
2- Lemperle G, Roman J, Busso M. Soft tissue augmentation whith artecoll: 10 year History. Indications, Thechniques, and Complications. Dermatol Surg 2003; 29: 573-587.
3- Barretto S, Lipodystrophy, Publisher Santos, 2007.
4- Reinaldo, J. S et al. Rheological, mechanical and morphological properties of polymethyl methacrylate / polyethylene terephthalate blend with dual reactive interfacial compatibilization. Polymers, Oct 2015, vol.25, no.5, p.451-460.
5- Dornelas, M. T et al. Lipodystrophy bioplasty in patients with HIV / AIDS. Bras. Cir. Plast. Sep 2012, vol.27, no.3, p.387-391.
6- Valero, M.F. et al. Physical-mechanical, thermal and morphological characterization of polyurethane-based interpenetrated lattice polymers obtained from acceptance of modified castor and almond / polymethyl methacrylate (PMMA). Polymers, 2011, vol.21, no.4, p.293-298
7- Campos, D. L. P. D., Proto, R. S., Santos, D. C. D., Oliveira Ruiz, R. D., Brancaccio, N., & Gonella, H. A. (2011). Histopathological evaluation of polymethylmethacrylate in rats for one year. Brazilian Journal of Plastic Surgery, 26 (2), 189-193.
Cohen SR, Holmes RE. Artecoll: a long lasting injectable wrinkle filler material: report of controlled, randomized, multicenter clinical trial of 251 subjects. Plast Reconstr Surg. 2004 Sep 15; 114 (4): 964-76.
8- Cohen JL. Understanding, avoiding and managing dermal filler complication. Dermatol Surg. 2008 Jun; 34 Suppl 1: S92-9.
9- Mc Clelland M., Egbert B., Hanko V., Berg R., and DeLustrus F. Evaluation of Artecoll Polymethylmethacrylate Implant for Soft-Tissue Augmentation: Biocompatibility and Chemical Characterization. Past Rebuild Surg. 100: 1466, 1997.
10- Cohen SR, Holmes RE. Artecoll: A Longlasting wrinkle augmentation material. To be published in Plast Reconstr Surg 113,2004.
11- Ersek, R.A. and Beisang, A.A., 3rd. Bioplastic: A new textured copolymer microparticle promises permanence in soft tissue augmentation. Plast Rebuild Surg. 87: 693, 1991.
12- Allen O, Response to subdermal implantation of textured microimplants in humans. Aesth Plast. Surg. 16: 227-230, 1992.
13- Rubin P., and Yaremcuk M .. Complications and Toxicities of Implantable Biomaterials Used in Facial Reconstructive and Aesthetic Surgery: A Comprehensive Review of the Literature. Plast Reconstr. Surg. 100: 1336, 1997.
14- Lemperle G., Legaz ME. Biocompatibility of injectable microparticles. Aesth Plast Surg. 2004
15- Garcez CE, Liquid Implants. In: Garcez CE, Arena-de-Souza R. Themes of Aesthetic Medicine 2 ed Porto Alegre: New Test, 2007. P.77-113.
16- Nácul AM. Contour of the Lower Third of the Face Using an Intramusculary Injectable Implant. Plast Surg. 29: 222–229, 2005.
17- Piacquadio D, Smith S, Anderson R. A comparison of commercially available polymethylmethacrylate-based soft tissue fillers. Dermatol Surg. 2008 Jun; 34 Suppl 1: S48-52.
18- Cohen SR, Berner CF, Busso M, Clopton P, Hamilton D, Roman JJ, et al. Five-year safety and efficacy of a novel polymethylmetacrylate aesthetic soft tissue filler for the correction of nasolabial folds. Dermatol Surg. 2007 Dec; 33 Suppl 2: S222-30.
19- Lemperle G., Morhenn V., and Charrier U. Human Histology and Persistence of Various Injectable Filler Substances for Soft Tissue Augmentation. Esthet. Plast Surg. 27: 354-366, 2003.
